The assembly of developing motor neurons depends on an interplay between spontaneous activity, type II cadherins and gap junctions
- PMID: 28246212
- PMCID: PMC5374350
- DOI: 10.1242/dev.144063
The assembly of developing motor neurons depends on an interplay between spontaneous activity, type II cadherins and gap junctions
Abstract
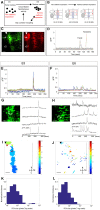
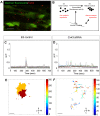
A core structural and functional motif of the vertebrate central nervous system is discrete clusters of neurons or 'nuclei'. Yet the developmental mechanisms underlying this fundamental mode of organisation are largely unknown. We have previously shown that the assembly of motor neurons into nuclei depends on cadherin-mediated adhesion. Here, we demonstrate that the emergence of mature topography among motor nuclei involves a novel interplay between spontaneous activity, cadherin expression and gap junction communication. We report that nuclei display spontaneous calcium transients, and that changes in the activity patterns coincide with the course of nucleogenesis. We also find that these activity patterns are disrupted by manipulating cadherin or gap junction expression. Furthermore, inhibition of activity disrupts nucleogenesis, suggesting that activity feeds back to maintain integrity among motor neurons within a nucleus. Our study suggests that a network of interactions between cadherins, gap junctions and spontaneous activity governs neuron assembly, presaging circuit formation.
Keywords: Brainstem; Cadherins; Cell adhesion; Chick; Gap junctions; Motor neurons; Spontaneous activity.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Benjumeda I., Escalante A., Law C., Morales D., Chauvin G., Muca G., Coca Y., Marquez J., Lopez-Bendito G., Kania A. et al. (2013). Uncoupling of EphA/ephrinA signaling and spontaneous activity in neural circuit wiring. J. Neurosci. 33, 18208-18218. 10.1523/JNEUROSCI.1931-13.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

