A novel CCR2 antagonist inhibits atherogenesis in apoE deficient mice by achieving high receptor occupancy
- PMID: 28246398
- PMCID: PMC5427923
- DOI: 10.1038/s41598-017-00104-z
A novel CCR2 antagonist inhibits atherogenesis in apoE deficient mice by achieving high receptor occupancy
Abstract
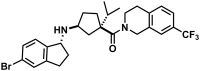
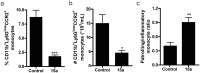
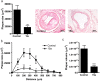
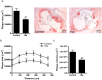
CC Chemokine Receptor 2 (CCR2) and its endogenous ligand CCL2 are involved in a number of diseases, including atherosclerosis. Several CCR2 antagonists have been developed as potential therapeutic agents, however their in vivo clinical efficacy was limited. In this report, we aimed to determine whether 15a, an antagonist with a long residence time on the human CCR2, is effective in inhibiting the development of atherosclerosis in a mouse disease model. First, radioligand binding assays were performed to determine affinity and binding kinetics of 15a on murine CCR2. To assess the in vivo efficacy, western-type diet fed apoE-/- mice were treated daily with 15a or vehicle as control. Treatment with 15a reduced the amount of circulating CCR2+ monocytes and the size of the atherosclerotic plaques in both the carotid artery and the aortic root. We then showed that the long pharmacokinetic half-life of 15a combined with the high drug concentrations ensured prolonged CCR2 occupancy. These data render 15a a promising compound for drug development and confirms high receptor occupancy as a key parameter when targeting chemokine receptors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

