Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis
- PMID: 28246548
- PMCID: PMC5305018
- DOI: 10.1177/1756283X16681708
Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis
Abstract
Background: Otilonium bromide (OB) is a spasmolytic agent acting as an L-type calcium channel antagonist in intestinal and colonic smooth muscle cells (SMCs). We analyzed three independent clinical trials with homogeneous design on patients with irritable bowel syndrome (IBS). After 2 weeks receiving placebo, patients were randomized to receive OB (3 × 40 mg daily) or placebo for 15 weeks. We aimed to perform a pooled analysis of the data from these homogeneous clinical trials to evaluate the efficacy of OB treatment on symptoms and global response of patients.
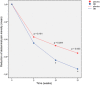
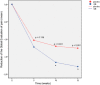
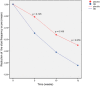
Methods: A total of 883 patients with IBS (69.8% women, mean age 46.2 years, 43.8% mixed type) were included, 442 treated with OB and 441 with placebo. The efficacy results from the three studies at weeks 5, 10 and 15 were pooled in an intention-to-treat (ITT) strategy, analyzed with a logistic regression model and described by forest plots.
Results: Despite a placebo effect in all efficacy variables, a significant therapeutic effect of OB was observed at weeks 10 and 15 with reference to: (a) intensity and frequency of abdominal pain; (b) rate of responders as evaluated by patients (71.8% at week 10 and 77.2% at week 15); (c) severity of bloating; (d) rate of responders as evaluated by physicians (55% at week 10 and 63.9% at week 15). No significant OB effect was observed in stool frequency and consistency.
Conclusions: OB is more effective than placebo in IBS treatment. Therapeutic benefits are significant after 10 weeks and are maximal after 15 weeks of treatment.
Keywords: abdominal pain; bloating; distention; global response; intention-to-treat analysis; irritable bowel syndrome; otilonium bromide.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome.Aliment Pharmacol Ther. 2011 Aug;34(4):432-42. doi: 10.1111/j.1365-2036.2011.04730.x. Epub 2011 Jun 16. Aliment Pharmacol Ther. 2011. PMID: 21679214 Clinical Trial.
-
Otilonium bromide in irritable bowel syndrome: a dose-ranging randomized double-blind placebo-controlled trial.World J Gastroenterol. 2014 Sep 14;20(34):12283-91. doi: 10.3748/wjg.v20.i34.12283. World J Gastroenterol. 2014. PMID: 25232263 Free PMC article. Clinical Trial.
-
The evaluation of otilonium bromide treatment in asian patients with irritable bowel syndrome.J Neurogastroenterol Motil. 2011 Oct;17(4):402-10. doi: 10.5056/jnm.2011.17.4.402. Epub 2011 Oct 31. J Neurogastroenterol Motil. 2011. PMID: 22148110 Free PMC article.
-
Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide.Therap Adv Gastroenterol. 2014 Jul;7(4):156-66. doi: 10.1177/1756283X14525250. Therap Adv Gastroenterol. 2014. PMID: 25057296 Free PMC article. Review.
-
Long-term efficacy and safety of otilonium bromide in the management of irritable bowel syndrome: a literature review.Clin Exp Gastroenterol. 2014 Apr 7;7:75-82. doi: 10.2147/CEG.S46291. eCollection 2014. Clin Exp Gastroenterol. 2014. PMID: 24741324 Free PMC article. Review.
Cited by
-
Irritable Bowel Syndrome Therapeutic Has Broad-Spectrum Antimicrobial Activity.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0044321. doi: 10.1128/AAC.00443-21. Epub 2021 Jul 19. Antimicrob Agents Chemother. 2021. PMID: 34280019 Free PMC article.
-
Neurogastroenterology and motility disorders in patients with cirrhosis.Hepatol Commun. 2025 Jan 7;9(1):e0622. doi: 10.1097/HC9.0000000000000622. eCollection 2025 Jan 1. Hepatol Commun. 2025. PMID: 39773873 Free PMC article. Review.
-
Diet or medication in primary care patients with IBS: the DOMINO study - a randomised trial supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute.Gut. 2022 Nov;71(11):2226-2232. doi: 10.1136/gutjnl-2021-325821. Epub 2022 Apr 28. Gut. 2022. PMID: 35483886 Free PMC article. Clinical Trial.
-
Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility.United European Gastroenterol J. 2022 Jul;10(6):556-584. doi: 10.1002/ueg2.12259. Epub 2022 Jun 13. United European Gastroenterol J. 2022. PMID: 35695704 Free PMC article. Review.
-
Effect of Samryungbaekchul-san Combined with Otilonium Bromide on Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Randomized Controlled Trial.J Clin Med. 2019 Sep 27;8(10):1558. doi: 10.3390/jcm8101558. J Clin Med. 2019. PMID: 31569833 Free PMC article.
References
-
- Agarwal N., Spiegel B. (2011) The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am 40: 11–19. - PubMed
-
- Baldi F., Longanesi A., Blasi A., Monello S., Cestari R., Missale G., et al. (1992) Octylonium bromide in the treatment of the irritable bowel syndrome: a clinical-functional study. Hepatogastroenterology 39: 392–395. - PubMed
-
- Battaglia G., Morselli-Labate A., Camarri E., Francavilla A., De Marco F., Mastropaolo G., et al. (1998) Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther 12: 1003–1010. - PubMed
-
- Boeckxstaens G., Corazziari E., Mearin F., Tack J. (2013) IBS and the role of otilonium bromide. Int J Colorectal Dis 28: 295–304. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

