Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis
- PMID: 28246548
- PMCID: PMC5305018
- DOI: 10.1177/1756283X16681708
Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis
Abstract
Background: Otilonium bromide (OB) is a spasmolytic agent acting as an L-type calcium channel antagonist in intestinal and colonic smooth muscle cells (SMCs). We analyzed three independent clinical trials with homogeneous design on patients with irritable bowel syndrome (IBS). After 2 weeks receiving placebo, patients were randomized to receive OB (3 × 40 mg daily) or placebo for 15 weeks. We aimed to perform a pooled analysis of the data from these homogeneous clinical trials to evaluate the efficacy of OB treatment on symptoms and global response of patients.
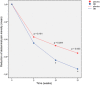
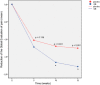
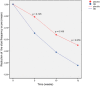
Methods: A total of 883 patients with IBS (69.8% women, mean age 46.2 years, 43.8% mixed type) were included, 442 treated with OB and 441 with placebo. The efficacy results from the three studies at weeks 5, 10 and 15 were pooled in an intention-to-treat (ITT) strategy, analyzed with a logistic regression model and described by forest plots.
Results: Despite a placebo effect in all efficacy variables, a significant therapeutic effect of OB was observed at weeks 10 and 15 with reference to: (a) intensity and frequency of abdominal pain; (b) rate of responders as evaluated by patients (71.8% at week 10 and 77.2% at week 15); (c) severity of bloating; (d) rate of responders as evaluated by physicians (55% at week 10 and 63.9% at week 15). No significant OB effect was observed in stool frequency and consistency.
Conclusions: OB is more effective than placebo in IBS treatment. Therapeutic benefits are significant after 10 weeks and are maximal after 15 weeks of treatment.
Keywords: abdominal pain; bloating; distention; global response; intention-to-treat analysis; irritable bowel syndrome; otilonium bromide.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



References
-
- Agarwal N., Spiegel B. (2011) The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am 40: 11–19. - PubMed
-
- Baldi F., Longanesi A., Blasi A., Monello S., Cestari R., Missale G., et al. (1992) Octylonium bromide in the treatment of the irritable bowel syndrome: a clinical-functional study. Hepatogastroenterology 39: 392–395. - PubMed
-
- Battaglia G., Morselli-Labate A., Camarri E., Francavilla A., De Marco F., Mastropaolo G., et al. (1998) Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther 12: 1003–1010. - PubMed
-
- Boeckxstaens G., Corazziari E., Mearin F., Tack J. (2013) IBS and the role of otilonium bromide. Int J Colorectal Dis 28: 295–304. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

