Impact of Combined Subthalamic Nucleus and Substantia Nigra Stimulation on Neuropsychiatric Symptoms in Parkinson's Disease Patients
- PMID: 28246572
- PMCID: PMC5299199
- DOI: 10.1155/2017/7306192
Impact of Combined Subthalamic Nucleus and Substantia Nigra Stimulation on Neuropsychiatric Symptoms in Parkinson's Disease Patients
Abstract
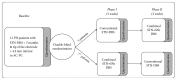
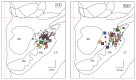
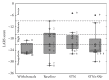
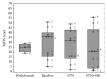
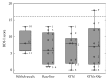
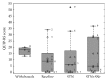
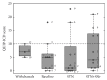
The goal of the study was to compare the tolerability and the effects of conventional subthalamic nucleus (STN) and combined subthalamic nucleus and substantia nigra (STN+SNr) high-frequency stimulation in regard to neuropsychiatric symptoms in Parkinson's disease patients. In this single center, randomized, double-blind, cross-over clinical trial, twelve patients with advanced Parkinson's disease (1 female; age: 61.3 ± 7.3 years; disease duration: 12.3 ± 5.4 years; Hoehn and Yahr stage: 2.2 ± 0.39) were included. Apathy, fatigue, depression, and impulse control disorder were assessed using a comprehensive set of standardized rating scales and questionnaires such as the Lille Apathy Rating Scale (LARS), Modified Fatigue Impact Scale (MFIS), Becks Depression Inventory (BDI-I), Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease Rating Scale (QUIP-RS), and Parkinson's Disease Questionnaire (PDQ-39). Three patients that were initially assigned to the STN+SNr stimulation mode withdrew from the study within the first week due to discomfort. Statistical comparison of data retrieved from patients who completed the study revealed no significant differences between both stimulation conditions in terms of mean scores of scales measuring apathy, fatigue, depression, impulse control disorder, and quality of life. Individual cases showed an improvement of apathy under combined STN+SNr stimulation. In general, combined STN+SNr stimulation seems to be safe in terms of neuropsychiatric side effects, although careful patient selection and monitoring in the short-term period after changing stimulation settings are recommended.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests. All authors declare no relevant conflict of interests. Some of the authors (U. Hidding, A. Gulberti, A. Horn, C. Buhmann, W. Hamel, C. K. E. Moll, and M. Pötter-Nerger) have occasionally been reimbursed for travel expenses from Medtronic Inc. C. Buhmann served on the scientific advisory board for GSK and UCB Pharma and received honoraria for lectures from GSK, Medtronic, Orion Pharma, and UCB. M. Pötter-Nerger served in advisory boards for Boston scientific, St. Jude, and AbbVie.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

