Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy
- PMID: 28246612
- PMCID: PMC5299189
- DOI: 10.1155/2017/4360357
Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy
Abstract
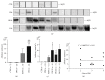
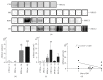
Diabetic nephropathy (DN) is a microangiopathic complication of diabetes mellitus (DM) affecting one-third of diabetic patients. The large variability in the clinical presentation of renal involvement in patients with DM makes kidney biopsy a prerequisite for a correct diagnosis. However, renal biopsy is an invasive procedure associated with risk of major complications. Numerous studies aimed to identify a noninvasive biomarker of DN but, so far, none of these is considered to be sufficiently specific and sensitive. Water channel aquaporins (AQPs), expressed at the plasma membrane of epithelial tubular cells, are often dysregulated during DN. In this work, we analyzed the urine excretion of AQP5 and AQP2 (uAQP5 and uAQP2), via exosomes, in 35 diabetic patients: 12 normoalbuminuric with normal renal function (DM), 11 with proteinuric nondiabetic nephropathy (NDN), and 12 with histological diagnosis and classification of DN. ELISA and WB analysis independently showed that uAQP5 was significantly increased in DN patients. Interestingly, linear regression analysis showed a positive correlation between uAQP5 and the histological class of DN. The same analysis, focusing on uAQP2, showed comparable results. Taken together, these data suggest a possible use of AQP5 and AQP2 as novel noninvasive biomarkers to help in classifying the clinical stage of DN.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Danaei G., Finucane M. M., Lu Y., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2∙7 million participants. The Lancet. 2011;378(9785):31–40. doi: 10.1016/s0140-6736(11)60679-x. - DOI - PubMed
-
- Collins A. J., Foley R. N., Chavers B., et al. United States Renal Data System 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. American Journal of Kidney Diseases. 2012;59(1, supplement 1, article A7):e1–e420. doi: 10.1053/j.ajkd.2011.11.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

