Effect of Fingolimod on Brain Volume Loss in Patients with Multiple Sclerosis
- PMID: 28247239
- PMCID: PMC5374177
- DOI: 10.1007/s40263-017-0415-2
Effect of Fingolimod on Brain Volume Loss in Patients with Multiple Sclerosis
Abstract
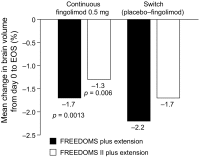
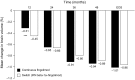
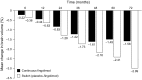
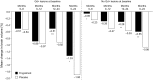
Brain atrophy occurs at a faster rate in patients with multiple sclerosis (MS) than in healthy individuals. In three randomized, controlled, phase III trials, fingolimod reduced the annual rate of brain volume loss (BVL) in patients with relapsing MS (RMS) by approximately one-third relative to that in individuals receiving placebo or intramuscular interferon beta-1a. Analysis of brain volume changes during study extensions has shown that this reduced rate of BVL is sustained in patients with RMS receiving fingolimod continuously. Subgroup analyses of the core phase III and extension studies have shown that reductions in the rate of BVL are observed irrespective of levels of inflammatory lesion activity seen by magnetic resonance imaging at baseline and on study; levels of disability at baseline; and treatment history. The rate of BVL in these studies was predicted independently by T2 lesion and gadolinium-enhancing lesion burdens at baseline, and correlations observed between BVL and increasing levels of disability strengthened over time. In another phase III trial in patients with primary progressive MS (PPMS), fingolimod did not reduce BVL overall relative to placebo; however, consistent with findings in RMS, there was a treatment effect on BVL in patients with PPMS with gadolinium-enhancing lesion activity at baseline. The association between treatment effects on BVL and future accumulation of disability argues in favor of measuring BVL on a more routine basis and with a more structured approach than is generally the case in clinical practice. Despite several practical obstacles, progress is being made in achieving this goal.
Conflict of interest statement
Funding
Editorial and medical writing support for the development of this manuscript was funded by Novartis Pharma AG. Journal open-access costs were also met by Novartis Pharma AG.
Conflicts of Interest
NDS has received honoraria from Biogen-Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Merck Serono, Novartis, Biogen-Idec, Roche, and Genzyme, and he has received research grant support from the Italian MS Society. DS is an employee of Novartis Pharma AG. MHB has received institutional support from Biogen, Genzyme, Merck, Novartis, and Teva, and is a research consultant for Medical Safety Systems.
Figures
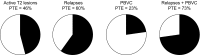
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

