The Barrett's Gland in Phenotype Space
- PMID: 28247864
- PMCID: PMC5301147
- DOI: 10.1016/j.jcmgh.2014.10.001
The Barrett's Gland in Phenotype Space
Abstract
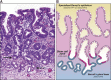
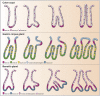
Barrett's esophagus is characterized by the erosive replacement of esophageal squamous epithelium by a range of metaplastic glandular phenotypes. These glandular phenotypes likely change over time, and their distribution varies along the Barrett's segment. Although much recent work has addressed Barrett's esophagus from the genomic viewpoint-its genotype space-the fact that the phenotype of Barrett's esophagus is nonstatic points to conversion between phenotypes and suggests that Barrett's esophagus also exists in phenotype space. Here we explore this latter concept, investigating the scope of glandular phenotypes in Barrett's esophagus and how they exist in physical and temporal space as well as their evolution and their life history. We conclude that individual Barrett's glands are clonal units; because of this important fact, we propose that it is the Barrett's gland that is the unit of selection in phenotypic and indeed neoplastic progression. Transition between metaplastic phenotypes may be governed by neutral drift akin to niche turnover in normal and dysplastic niches. In consequence, the phenotype of Barrett's glands assumes considerable importance, and we make a strong plea for the integration of the Barrett's gland in both genotype and phenotype space in future work.
Keywords: Barrett’s Esophagus; CCO, cytochrome c oxidase; Metaplasia; Neutral Drift.
Figures


References
-
- Maley C.C., Galipeau P.C., Li X. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res. 2004;64:3414–3427. - PubMed
-
- Bansal A., McGregor D.H., Anand O. Presence or absence of intestinal metaplasia but not its burden is associated with prevalent high-grade dysplasia and cancer in Barrett’s esophagus. Dis Esophagus. 2014;27:751–756. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

