Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma
- PMID: 28248126
- PMCID: PMC5477829
- DOI: 10.1200/JCO.2016.67.1222
Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma
Abstract
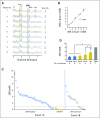
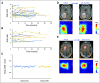
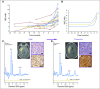
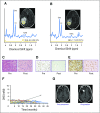
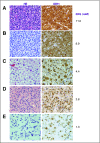
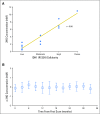
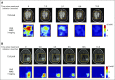
Purpose Proton magnetic resonance spectroscopy (MRS) of the brain can detect 2-hydroxyglutarate (2HG), the oncometabolite produced in neoplasms harboring a mutation in the gene coding for isocitrate dehydrogenase ( IDH). We conducted a prospective longitudinal imaging study to determine whether quantitative assessment of 2HG by MRS could serve as a noninvasive clinical imaging biomarker for IDH-mutated gliomas. Patients and Methods 2HG MRS was performed in 136 patients using point-resolved spectroscopy at 3 T in parallel with standard clinical magnetic resonance imaging and assessment. Data were analyzed in patient cohorts representing the major phases of the glioma clinical course and were further subgrouped by histology and treatment type to evaluate 2HG. Histologic correlations were performed. Results Quantitative 2HG MRS was technically and biologically reproducible. 2HG concentration > 1 mM could be reliably detected with high confidence. During the period of indolent disease, 2HG concentration varied by less than ± 1 mM, and it increased sharply with tumor progression. 2HG concentration was positively correlated with tumor cellularity and significantly differed between high- and lower-grade gliomas. In response to cytotoxic therapy, 2HG concentration decreased rapidly in 1p/19q codeleted oligodendrogliomas and with a slower time course in astrocytomas and mixed gliomas. The magnitude and time course of the decrease in 2HG concentration and magnitude of the decrease in tumor volume did not differ between oligodendrogliomas treated with temozolomide or carmustine. Criteria for 2HG MRS were established to make a presumptive molecular diagnosis of an IDH mutation in gliomas technically unable to undergo a surgical procedure. Conclusion 2HG concentration as measured by MRS was reproducible and reliably reflected the disease state. These data provide a basis for incorporating 2HG MRS into clinical management of IDH-mutated gliomas.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

