A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility
- PMID: 28248294
- PMCID: PMC5336136
- DOI: 10.1038/nmicrobiol.2017.7
A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility
Abstract
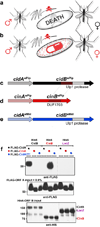
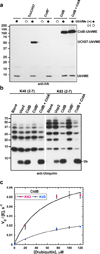
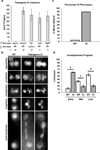
Wolbachia are obligate intracellular bacteria1 that infect arthropods, including approximately two-thirds of insect species2. Wolbachia manipulate insect reproduction by enhancing their inheritance through the female germline. The most common alteration is cytoplasmic incompatibility (CI)3-5, where eggs from uninfected females fail to develop when fertilized by sperm from Wolbachia-infected males. By contrast, if female and male partners are both infected, embryos are viable. CI is a gene-drive mechanism impacting population structure6 and causing reproductive isolation7, but its molecular mechanism has remained unknown. We show that a Wolbachia deubiquitylating enzyme (DUB) induces CI. The CI-inducing DUB, CidB, cleaves ubiquitin from substrates and is encoded in a two-gene operon, and the other protein, CidA, binds CidB. Binding is strongest between cognate partners in cidA-cidB homologues. In transgenic Drosophila, the cidA-cidB operon mimics CI when sperm introduce it into eggs, and a catalytically inactive DUB does not induce sterility. Toxicity is recapitulated in yeast by CidB alone; this requires DUB activity but is rescued by coexpressed CidA. A paralogous operon involves a putative nuclease (CinB) rather than a DUB. Analogous binding, toxicity and rescue in yeast were observed. These results identify a CI mechanism involving interacting proteins that are secreted into germline cells by Wolbachia, and suggest new methods for insect control.
Figures

Comment in
-
Microbiology: Manipulation of the manipulators.Nature. 2017 Mar 9;543(7644):182-183. doi: 10.1038/nature21509. Epub 2017 Feb 26. Nature. 2017. PMID: 28241145 No abstract available.
-
Symbiosis: Wolbachia's matchmaking secret revealed.Nat Rev Microbiol. 2017 Mar 13;15(4):194-195. doi: 10.1038/nrmicro.2017.25. Nat Rev Microbiol. 2017. PMID: 28286343 No abstract available.
References
-
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. - PubMed
-
- Laven H. Chapter 7: Speciation and Evolution in Culex pipiens. Elselvier; 1967. p. 251.
-
- Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature. 1971;232:657–658. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

