Phenylthiazole Antibacterial Agents Targeting Cell Wall Synthesis Exhibit Potent Activity in Vitro and in Vivo against Vancomycin-Resistant Enterococci
- PMID: 28248504
- PMCID: PMC5437844
- DOI: 10.1021/acs.jmedchem.6b01780
Phenylthiazole Antibacterial Agents Targeting Cell Wall Synthesis Exhibit Potent Activity in Vitro and in Vivo against Vancomycin-Resistant Enterococci
Abstract
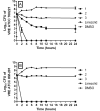
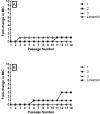
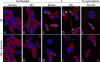
The emergence of antibiotic-resistant bacterial species, such as vancomycin-resistant enterococci (VRE), necessitates the development of new antimicrobials. Here, we investigate the spectrum of antibacterial activity of three phenylthiazole-substituted aminoguanidines. These compounds possess potent activity against VRE, inhibiting growth of clinical isolates at concentrations as low as 0.5 μg/mL. The compounds exerted a rapid bactericidal effect, targeting cell wall synthesis. Transposon mutagenesis suggested three possible targets: YubA, YubB (undecaprenyl diphosphate phosphatase (UPPP)), and YubD. Both UPPP as well as undecaprenyl diphosphate synthase were inhibited by compound 1. YubA and YubD are annotated as transporters and may also be targets because 1 collapsed the proton motive force in membrane vesicles. Using Caenorhabditis elegans, we demonstrate that two compounds (1, 3, at 20 μg/mL) retain potent activity in vivo, significantly reducing the burden of VRE in infected worms. Taken altogether, the results indicate that compounds 1 and 3 warrant further investigation as novel antibacterial agents against drug-resistant enterococci.
Figures
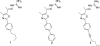


Similar articles
-
Discovery of Gambogic acid as an antibacterial adjuvant against vancomycin-resistant enterococci in vitro and in vivo.Phytomedicine. 2024 Jun;128:155400. doi: 10.1016/j.phymed.2024.155400. Epub 2024 Feb 1. Phytomedicine. 2024. PMID: 38518641
-
Alkoxyphenylthiazoles with broad-spectrum activity against multidrug-resistant gram-positive bacterial pathogens.Eur J Med Chem. 2018 May 25;152:318-328. doi: 10.1016/j.ejmech.2018.04.049. Epub 2018 Apr 25. Eur J Med Chem. 2018. PMID: 29734000
-
Oritavancin Combinations with β-Lactams against Multidrug-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci.Antimicrob Agents Chemother. 2016 Mar 25;60(4):2352-8. doi: 10.1128/AAC.03006-15. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 26833159 Free PMC article.
-
Treatment options for vancomycin-resistant enterococcal infections.Drugs. 2002;62(3):425-41. doi: 10.2165/00003495-200262030-00002. Drugs. 2002. PMID: 11827558 Review.
-
Therapeutic options for vancomycin-resistant enterococcal bacteremia.Expert Rev Anti Infect Ther. 2015 Mar;13(3):363-77. doi: 10.1586/14787210.2015.1001839. Expert Rev Anti Infect Ther. 2015. PMID: 25661903 Review.
Cited by
-
Aromatic Guanylhydrazones for the Control of Heme-Induced Antibody Polyreactivity.ACS Omega. 2019 Nov 22;4(24):20450-20458. doi: 10.1021/acsomega.9b01548. eCollection 2019 Dec 10. ACS Omega. 2019. PMID: 31858028 Free PMC article.
-
tert-Butylphenylthiazoles with an oxadiazole linker: a novel orally bioavailable class of antibiotics exhibiting antibiofilm activity.RSC Adv. 2019 Feb 26;9(12):6770-6778. doi: 10.1039/c8ra10525a. eCollection 2019 Feb 22. RSC Adv. 2019. PMID: 35518469 Free PMC article.
-
Synthesis, Antimicrobial Activities, and Model of Action of Novel Tetralone Derivatives Containing Aminoguanidinium Moiety.Int J Mol Sci. 2025 Jun 21;26(13):5980. doi: 10.3390/ijms26135980. Int J Mol Sci. 2025. PMID: 40649759 Free PMC article.
-
Antibacterial and antivirulence activities of auranofin against Clostridium difficile.Int J Antimicrob Agents. 2019 Jan;53(1):54-62. doi: 10.1016/j.ijantimicag.2018.09.018. Epub 2018 Sep 28. Int J Antimicrob Agents. 2019. PMID: 30273668 Free PMC article.
-
Potential Antimicrobial Isopropanol-Conjugated Carbazole Azoles as Dual Targeting Inhibitors of Enterococcus faecalis.ACS Med Chem Lett. 2018 Feb 5;9(3):244-249. doi: 10.1021/acsmedchemlett.7b00514. eCollection 2018 Mar 8. ACS Med Chem Lett. 2018. PMID: 29541368 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Preventing healthcare-associated infections. 2016 Dec 5; http://www.cdc.gov/washington/~cdcatWork/pdf/infections.pdf.
-
- World Health Organization. Antimicrobial resistance global report on surveillance 2014. WHO Press; France: 2014. pp. 1–232.
-
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta: CDC; 2013. pp. 1–114.
-
- Patel R. Clinical impact of vancomycin-resistant enterococci. J Antimicrob Chemother. 2003;51(Suppl 3):iii13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

