Visible-Light Excitation of a Molecular Motor with an Extended Aromatic Core
- PMID: 28248510
- PMCID: PMC5359586
- DOI: 10.1021/acs.orglett.7b00317
Visible-Light Excitation of a Molecular Motor with an Extended Aromatic Core
Abstract
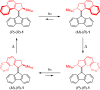
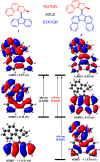
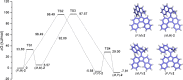
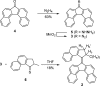
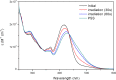
Exploring routes to visible-light-driven rotary motors, the possibility of red-shifting the excitation wavelength of molecular motors by extension of the aromatic core is studied. Introducing a dibenzofluorenyl moiety in a standard molecular motor resulted in red-shifting of the absorption spectrum. UV/vis and 1H NMR spectroscopy showed that these motors could be isomerized with light of wavelengths up to 490 nm and that the structural modification did not impair the anticipated rotary behavior. Extension of the aromatic core is therefore a suitable strategy to apply in pursuit of visible-light-driven molecular motors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Light-Gated Rotation in a Molecular Motor Functionalized with a Dithienylethene Switch.Angew Chem Int Ed Engl. 2018 Aug 13;57(33):10515-10519. doi: 10.1002/anie.201802392. Epub 2018 Jun 15. Angew Chem Int Ed Engl. 2018. PMID: 29806875 Free PMC article.
-
Driving unidirectional molecular rotary motors with visible light by intra- and intermolecular energy transfer from palladium porphyrin.J Am Chem Soc. 2012 Oct 24;134(42):17613-9. doi: 10.1021/ja306986g. Epub 2012 Oct 16. J Am Chem Soc. 2012. PMID: 23036108
-
Photoefficient 2nd generation molecular motors responsive to visible light.Chem Sci. 2019 Aug 9;10(38):8768-8773. doi: 10.1039/c9sc02150g. eCollection 2019 Oct 14. Chem Sci. 2019. PMID: 31803449 Free PMC article.
-
Visible Light-Driven Molecular Switches and Motors: Recent Developments and Applications.Chemistry. 2022 Mar 28;28(18):e202103906. doi: 10.1002/chem.202103906. Epub 2022 Jan 28. Chemistry. 2022. PMID: 34964995 Review.
-
Designing light-driven rotary molecular motors.Chem Sci. 2021 Oct 20;12(45):14964-14986. doi: 10.1039/d1sc04781g. eCollection 2021 Nov 24. Chem Sci. 2021. PMID: 34909140 Free PMC article. Review.
Cited by
-
Molecular rotary motors: Unidirectional motion around double bonds.Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):9423-9431. doi: 10.1073/pnas.1712784115. Epub 2018 Apr 30. Proc Natl Acad Sci U S A. 2018. PMID: 29712825 Free PMC article.
-
Designing P-type bi-stable overcrowded alkene-based chiroptical photoswitches.Chem Sci. 2023 Mar 13;14(16):4328-4336. doi: 10.1039/d2sc05903g. eCollection 2023 Apr 26. Chem Sci. 2023. PMID: 37123178 Free PMC article.
-
Adsorption and Self-Aggregation of Chiral [5]-Aza[6]helicenes on DNA Architecture: A Molecular Dynamics Study.J Phys Chem B. 2023 Oct 5;127(39):8285-8295. doi: 10.1021/acs.jpcb.3c02487. Epub 2023 Sep 26. J Phys Chem B. 2023. PMID: 37751596 Free PMC article.
-
Directing Coupled Motion with Light: A Key Step Toward Machine-Like Function.Chem Rev. 2021 Nov 10;121(21):13213-13237. doi: 10.1021/acs.chemrev.1c00340. Epub 2021 Sep 17. Chem Rev. 2021. PMID: 34533944 Free PMC article.
-
Coupling Rotary Motion to Helicene Inversion within a Molecular Motor.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202416097. doi: 10.1002/anie.202416097. Epub 2024 Nov 26. Angew Chem Int Ed Engl. 2025. PMID: 39526696 Free PMC article.
References
-
- Molecular Switches; Browne W. R., Feringa B. L., Eds.; Wiley-VCH: Weinheim, 2011.
- From Non-Covalent Assemblies to Molecular Machines; Sauvage J. P., Gaspard P., Eds; Wiley-VCH: Weinheim, 2010.
- Molecular Devices and Machines: Concepts and Perspectives for the Nanoworld; Balzani V., Credi A., Venturi M., Eds.; Wiley-VCH: Weinheim, 2008.
- Cheng C.; Stoddart J. F. ChemPhysChem 2016, 17, 1780.10.1002/cphc.201501155. - DOI - PubMed
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Chem. Rev. 2015, 115, 10081.10.1021/acs.chemrev.5b00146. - DOI - PMC - PubMed
- Coskun A.; Banaszak M.; Astumian R. D.; Stoddart J. F.; Grzybowski B. A. Chem. Soc. Rev. 2012, 41, 19.10.1039/C1CS15262A. - DOI - PubMed
- Kinbara K.; Aida T. Chem. Rev. 2005, 105, 1377.10.1021/cr030071r. - DOI - PubMed
-
- Kundu P. K.; Klajn R. ACS Nano 2014, 8, 11913.10.1021/nn506656r. - DOI - PubMed
- Spruell J. M.; Hawker C. J. Chem. Sci. 2011, 2, 18.10.1039/C0SC00426J. - DOI
- Qu D. H.; Wang Q. C.; Zhang Q. W.; Ma X.; Tian H. Chem. Rev. 2015, 115, 7543.10.1021/cr5006342. - DOI - PubMed
- Klajn R. Chem. Soc. Rev. 2014, 43, 148.10.1039/C3CS60181A. - DOI - PubMed
- Zhang J.; Zou Q.; Tian H. Adv. Mater. 2013, 25, 378.10.1002/adma.201201521. - DOI - PubMed
- Russew M. M.; Hecht S. Adv. Mater. 2010, 22, 3348.10.1002/adma.200904102. - DOI - PubMed
-
- Broichhagen J.; Frank J. A.; Trauner D. Acc. Chem. Res. 2015, 48, 1947.10.1021/acs.accounts.5b00129. - DOI - PubMed
- Dong M.; Babalhavaeji A.; Samanta S.; Beharry A. A.; Woolley G. A. Acc. Chem. Res. 2015, 48, 2662.10.1021/acs.accounts.5b00270. - DOI - PubMed
- Lerch M. M.; Hansen M. J.; van Dam G. M.; Szymanski W.; Feringa B. L. Angew. Chem., Int. Ed. 2016, 55, 10978.10.1002/anie.201601931. - DOI - PubMed
- Velema W. A.; Szymanski W.; Feringa B. L. J. Am. Chem. Soc. 2014, 136, 2178.10.1021/ja413063e. - DOI - PubMed
-
- Beharry A. A.; Sadovski O.; Woolley G. A. J. Am. Chem. Soc. 2011, 133, 19684.10.1021/ja209239m. - DOI - PubMed
- Bléger D.; Schwarz J.; Brouwer A. M.; Hecht S. J. Am. Chem. Soc. 2012, 134, 20597.10.1021/ja310323y. - DOI - PubMed
- Konrad D. B.; Frank J. A.; Trauner D. Chem. - Eur. J. 2016, 22, 4364.10.1002/chem.201505061. - DOI - PubMed
- Samanta S.; McCormick T. M.; Schmidt S. K.; Seferos D. S.; Woolley G. A. Chem. Commun. 2013, 49, 10314.10.1039/c3cc46045b. - DOI - PubMed
- Hansen M. J.; Lerch M. M.; Szymanski W.; Feringa B. L. Angew. Chem., Int. Ed. 2016, 55, 13514.10.1002/anie.201607529. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

