Long-term Behavior of Serous Borderline Tumors Subdivided Into Atypical Proliferative Tumors and Noninvasive Low-grade Carcinomas: A Population-based Clinicopathologic Study of 942 Cases
- PMID: 28248817
- PMCID: PMC5423818
- DOI: 10.1097/PAS.0000000000000824
Long-term Behavior of Serous Borderline Tumors Subdivided Into Atypical Proliferative Tumors and Noninvasive Low-grade Carcinomas: A Population-based Clinicopathologic Study of 942 Cases
Abstract
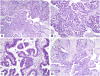
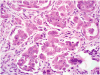
Ovarian serous borderline tumors (SBTs) have been the subject of considerable controversy, particularly with regard to terminology and behavior. It has been proposed that they constitute a heterogenous group of tumors composed, for the most part, of typical SBTs that are benign and designated "atypical proliferative serous tumor (APST)" and a small subset of SBTs with micropapillary architecture that have a poor outcome and are designated "noninvasive low-grade serous carcinoma (niLGSC)". It also has been argued that the difference in behavior between the 2 groups is not due to the subtype of the primary tumor but rather the presence of extraovarian disease, specifically invasive implants. According to the terminology of the 2014 WHO Classification, typical SBTs are equivalent to APSTs and SBTs displaying micropapillary architecture are synonymous with niLGSC. In addition, "invasive implants" were renamed "low-grade serous carcinoma" (LGSC). The argument as to whether it is the appearance of the primary tumor or the presence of extraovarian LGSC that determines outcome remains unsettled. The current study was initiated in 2004 and was designed to determine what factors were predictive of outcome, with special attention to the appearance of the primary tumor (APST vs. niLGSC) and that of the extraovarian disease (noninvasive vs. invasive implants). Our study is population based, involving the entire female population of Denmark. None of the women in the study were lost to follow-up, which ranged up to 36 years (median, 15 y). All the microscopic slides from the contributing hospitals were rereviewed by a panel of 2 pathologists (R.V. and R.J.K.) who were blinded to the follow-up. After excluding those that were not SBTs by the pathology panel, as well as cases with a prior or concurrent cancer or undefined stage, 942 women remained, of which 867 were APSTs and 75 were niLGSCs. The median patient age was 50 years (range, 16 to 97 y). Eight hundred nine women (86%) presented with FIGO stage I disease, whereas 133 (14%) had advanced stage disease. Compared with APSTs, niLGSC exhibited a significantly greater frequency of bilaterality, residual gross disease after surgery, microinvasion/microinvasive carcinoma, advanced stage disease, and invasive implants at presentation (P-values <0.003). Because the cause of death is difficult to accurately ascertain from death certificates, we used development of invasive serous carcinoma as the primary endpoint as following development of carcinoma, the mortality is very high. In the entire cohort, subsequent development of carcinoma occurred in 4%, of which 93% were low grade and 7% high grade (median time, 10 y; range, up to 25 y). After adjusting for age at and time since diagnosis of APST or niLGSC, occurrence of subsequent carcinoma was significantly higher with niLGSC than APST among all stages combined (hazard ratio [HR]=3.8; 95% confidence interval [CI], 1.7-8.2). This difference was still significant for stage I but not advanced stage cases. Moreover, all-cause mortality was not statistically significantly different between APST and niLGSC. Of all women with advanced stage disease, 114 (86%) had noninvasive implants, whereas 19 (14%) were invasive. Noninvasive implants were significantly associated with subsequent development of carcinoma (HR=7.7; 95% CI, 3.9-15.0), but the risk with invasive implants was significantly higher (HR=42.3; 95% CI, 16.1-111.1). In conclusion, although invasive implants are the most important feature in predicting an adverse outcome, subclassification into APST and niLGSC is important as it stratifies women with respect to risk for advanced stage disease and invasive implants for all women and development of serous carcinoma for stage I cases.
Figures







Similar articles
-
Clinicopathologic and Molecular Features of Paired Cases of Metachronous Ovarian Serous Borderline Tumor and Subsequent Serous Carcinoma.Am J Surg Pathol. 2019 Nov;43(11):1462-1472. doi: 10.1097/PAS.0000000000001325. Am J Surg Pathol. 2019. PMID: 31343420 Free PMC article.
-
A nationwide study of ovarian serous borderline tumors in Denmark 1978-2002. Risk of recurrence, and development of ovarian serous carcinoma.Gynecol Oncol. 2017 Jan;144(1):174-180. doi: 10.1016/j.ygyno.2016.11.007. Epub 2016 Nov 9. Gynecol Oncol. 2017. PMID: 27836204 Free PMC article.
-
Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion.Am J Surg Pathol. 2002 Sep;26(9):1111-28. doi: 10.1097/00000478-200209000-00002. Am J Surg Pathol. 2002. PMID: 12218568
-
Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas.Am J Surg Pathol. 2002 Dec;26(12):1529-41. doi: 10.1097/00000478-200212000-00001. Am J Surg Pathol. 2002. PMID: 12459620 Review.
-
Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion.Am J Surg Pathol. 2006 Oct;30(10):1209-21. doi: 10.1097/01.pas.0000213299.11649.fa. Am J Surg Pathol. 2006. PMID: 17001150 Review.
Cited by
-
Folate receptor alpha expression in low-grade serous ovarian cancer: Exploring new therapeutic possibilities.Gynecol Oncol. 2024 Sep;188:52-57. doi: 10.1016/j.ygyno.2024.06.008. Epub 2024 Jun 27. Gynecol Oncol. 2024. PMID: 38941962 Free PMC article.
-
MALDI-MSI-A Step Forward in Overcoming the Diagnostic Challenges in Ovarian Tumors.Int J Environ Res Public Health. 2020 Oct 18;17(20):7564. doi: 10.3390/ijerph17207564. Int J Environ Res Public Health. 2020. PMID: 33080944 Free PMC article.
-
Eosinophilic Cells as a Distinct Morphological Feature in BRAFV600E-Mutated Ovarian Serous Borderline Tumors.Diagnostics (Basel). 2025 Jun 11;15(12):1479. doi: 10.3390/diagnostics15121479. Diagnostics (Basel). 2025. PMID: 40564801 Free PMC article.
-
Mutation of NRAS is a rare genetic event in ovarian low-grade serous carcinoma.Hum Pathol. 2017 Oct;68:87-91. doi: 10.1016/j.humpath.2017.08.021. Epub 2017 Sep 2. Hum Pathol. 2017. PMID: 28873354 Free PMC article.
-
Prognosis and Prognostic Factors of Serous Borderline Tumor-Micropapillary Variant: Retrospective Study of 200 Patients with Long-Term Follow-Up.J Oncol. 2022 Oct 10;2022:1655422. doi: 10.1155/2022/1655422. eCollection 2022. J Oncol. 2022. PMID: 36262351 Free PMC article.
References
-
- Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors. Histologic features and prognosis. Cancer. 1988;62:2212–2222. - PubMed
-
- Bell DA, Longacre TA, Prat J, et al. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol. 2004;35:934–948. - PubMed
-
- Bell KA, Smith Sehdev AE, Kurman RJ. Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol. 2001;25:419–432. - PubMed
-
- Kurman RJ, Trimble CL. The behavior of serous tumors of low malignant potential: are they ever malignant? Int J Gynecol Pathol. 1993;12:120–127. - PubMed
-
- Seidman JD, Bell DA, Crum CP, et al. Tumours of the ovary: Epithelial tumours-Serous tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, et al., editors. WHO Classification of Tumours of Female Reproductive Organs. 4. Lyon, France: IARC Press; 2014. pp. 17–24.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

