A magnesium-induced triplex pre-organizes the SAM-II riboswitch
- PMID: 28248966
- PMCID: PMC5352136
- DOI: 10.1371/journal.pcbi.1005406
A magnesium-induced triplex pre-organizes the SAM-II riboswitch
Abstract
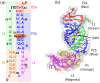
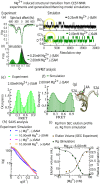
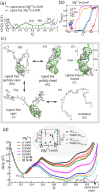
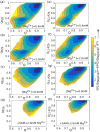
Our 13C- and 1H-chemical exchange saturation transfer (CEST) experiments previously revealed a dynamic exchange between partially closed and open conformations of the SAM-II riboswitch in the absence of ligand. Here, all-atom structure-based molecular simulations, with the electrostatic effects of Manning counter-ion condensation and explicit magnesium ions are employed to calculate the folding free energy landscape of the SAM-II riboswitch. We use this analysis to predict that magnesium ions remodel the landscape, shifting the equilibrium away from the extended, partially unfolded state towards a compact, pre-organized conformation that resembles the ligand-bound state. Our CEST and SAXS experiments, at different magnesium ion concentrations, quantitatively confirm our simulation results, demonstrating that magnesium ions induce collapse and pre-organization. Agreement between theory and experiment bolsters microscopic interpretation of our simulations, which shows that triplex formation between helix P2b and loop L1 is highly sensitive to magnesium and plays a key role in pre-organization. Pre-organization of the SAM-II riboswitch allows rapid detection of ligand with high selectivity, which is important for biological function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Cooperation between Magnesium and Metabolite Controls Collapse of the SAM-I Riboswitch.Biophys J. 2017 Jul 25;113(2):348-359. doi: 10.1016/j.bpj.2017.06.044. Biophys J. 2017. PMID: 28746845 Free PMC article.
-
Magnesium ions mitigate metastable states in the regulatory landscape of mRNA elements.RNA. 2024 Jul 16;30(8):992-1010. doi: 10.1261/rna.079767.123. RNA. 2024. PMID: 38777381 Free PMC article.
-
The expression platform and the aptamer: cooperativity between Mg2+ and ligand in the SAM-I riboswitch.Nucleic Acids Res. 2013 Feb 1;41(3):1922-35. doi: 10.1093/nar/gks978. Epub 2012 Dec 20. Nucleic Acids Res. 2013. PMID: 23258703 Free PMC article.
-
Modulation of Conformational Equilibria in the S-Adenosylmethionine (SAM) II Riboswitch by SAM, Mg(2+), and Trimethylamine N-Oxide.Biochemistry. 2016 Sep 13;55(36):5010-20. doi: 10.1021/acs.biochem.6b00283. Epub 2016 Sep 2. Biochemistry. 2016. PMID: 27552169
-
Magnesium ions mediate ligand binding and conformational transition of the SAM/SAH riboswitch.Commun Biol. 2023 Jul 31;6(1):791. doi: 10.1038/s42003-023-05175-5. Commun Biol. 2023. PMID: 37524918 Free PMC article.
Cited by
-
Structural insights into translation regulation by the THF-II riboswitch.Nucleic Acids Res. 2023 Jan 25;51(2):952-965. doi: 10.1093/nar/gkac1257. Nucleic Acids Res. 2023. PMID: 36620887 Free PMC article.
-
Exploring the Energy Landscape of Riboswitches Using Collective Variables Based on Tertiary Contacts.J Mol Biol. 2022 Sep 30;434(18):167788. doi: 10.1016/j.jmb.2022.167788. Epub 2022 Aug 11. J Mol Biol. 2022. PMID: 35963460 Free PMC article.
-
Deciphering ligand and metal ion dependent intricate folding landscape of Vc2 c-di-GMP riboswitch aptamer.Nucleic Acids Res. 2025 Jan 7;53(1):gkae1296. doi: 10.1093/nar/gkae1296. Nucleic Acids Res. 2025. PMID: 39777471 Free PMC article.
-
Magnesium controls aptamer-expression platform switching in the SAM-I riboswitch.Nucleic Acids Res. 2019 Apr 8;47(6):3158-3170. doi: 10.1093/nar/gky1311. Nucleic Acids Res. 2019. PMID: 30605518 Free PMC article.
-
Chelated Magnesium Logic Gate Regulates Riboswitch Pseudoknot Formation.J Phys Chem B. 2021 Jun 24;125(24):6479-6490. doi: 10.1021/acs.jpcb.1c02467. Epub 2021 Jun 9. J Phys Chem B. 2021. PMID: 34106719 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

