Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia
- PMID: 28249141
- PMCID: PMC5881572
- DOI: 10.1056/NEJMoa1609783
Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia
Abstract
Background: Blinatumomab, a bispecific monoclonal antibody construct that enables CD3-positive T cells to recognize and eliminate CD19-positive acute lymphoblastic leukemia (ALL) blasts, was approved for use in patients with relapsed or refractory B-cell precursor ALL on the basis of single-group trials that showed efficacy and manageable toxic effects.
Methods: In this multi-institutional phase 3 trial, we randomly assigned adults with heavily pretreated B-cell precursor ALL, in a 2:1 ratio, to receive either blinatumomab or standard-of-care chemotherapy. The primary end point was overall survival.
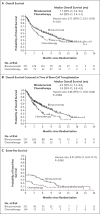
Results: Of the 405 patients who were randomly assigned to receive blinatumomab (271 patients) or chemotherapy (134 patients), 376 patients received at least one dose. Overall survival was significantly longer in the blinatumomab group than in the chemotherapy group. The median overall survival was 7.7 months in the blinatumomab group and 4.0 months in the chemotherapy group (hazard ratio for death with blinatumomab vs. chemotherapy, 0.71; 95% confidence interval [CI], 0.55 to 0.93; P=0.01). Remission rates within 12 weeks after treatment initiation were significantly higher in the blinatumomab group than in the chemotherapy group, both with respect to complete remission with full hematologic recovery (34% vs. 16%, P<0.001) and with respect to complete remission with full, partial, or incomplete hematologic recovery (44% vs. 25%, P<0.001). Treatment with blinatumomab resulted in a higher rate of event-free survival than that with chemotherapy (6-month estimates, 31% vs. 12%; hazard ratio for an event of relapse after achieving a complete remission with full, partial, or incomplete hematologic recovery, or death, 0.55; 95% CI, 0.43 to 0.71; P<0.001), as well as a longer median duration of remission (7.3 vs. 4.6 months). A total of 24% of the patients in each treatment group underwent allogeneic stem-cell transplantation. Adverse events of grade 3 or higher were reported in 87% of the patients in the blinatumomab group and in 92% of the patients in the chemotherapy group.
Conclusions: Treatment with blinatumomab resulted in significantly longer overall survival than chemotherapy among adult patients with relapsed or refractory B-cell precursor ALL. (Funded by Amgen; TOWER ClinicalTrials.gov number, NCT02013167 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Targeted therapies: New standard for relapsed ALL.Nat Rev Clin Oncol. 2017 May;14(5):264. doi: 10.1038/nrclinonc.2017.42. Epub 2017 Mar 21. Nat Rev Clin Oncol. 2017. PMID: 28322244 No abstract available.
-
Blinatumomab for Acute Lymphoblastic Leukemia.N Engl J Med. 2017 Jun 8;376(23):e49. doi: 10.1056/NEJMc1704012. N Engl J Med. 2017. PMID: 28594155 No abstract available.
References
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–43. - PubMed
-
- Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–801. - PubMed
-
- Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–25. - PubMed
-
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
