Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease
- PMID: 28249275
- PMCID: PMC6595218
- DOI: 10.1159/000450908
Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease
Abstract
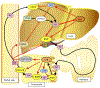
Farnesoid X receptor (FXR) regulates the synthesis, transport and enterohepatic circulation of bile acids (BA) by modulating the expression of related genes in the liver and small intestine. The composition of the gut microbiota is correlated with metabolic diseases, notably obesity and non-alcoholic fatty acid disease (NAFLD). Recent studies revealed that bacterial metabolism of BA can modulate FXR signaling in the intestine by altering the composition and concentrations of FXR agonist and antagonist. FXR agonist enhances while FXR antagonist suppresses obesity, NAFLD and insulin resistance. The role of intestinal FXR in metabolic disease was firmly established by the analysis of mice lacking FXR that are metabolic resistant to HFD-induced metabolic disease. This is mediated by FXR modulating in part the expression of genes involved in ceramide synthesis in the small intestine. In ileum of obese mice due to the presence of endogenous FXR agonists produced in the liver, these genes are activated, while in mice with altered levels of specific gut bacteria, levels of an FXR antagonist, tauro-β-muricholic acid (T-β-MCA) increase and FXR signaling and ceramide synthesis are repressed. T-β-MCA, which is metabolized in wild-type mice, led to the discovery of glycine-β-muricholic acid (Gly-MCA) that is stable in the intestine and a potent inhibitor of FXR signaling. These studies reveal that ceramides produced in the ileum under the control of FXR, influence metabolic disease, and suggest that novel FXR antagonist such as Gly-MCA that specifically inhibit intestine FXR, could serve as potential drug for the treatment of metabolic disease.
© 2017 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
A.D. Patterson owns equity in Heliome Biotech. This financial interest has been reviewed by the University’s Individual Conflict of Interest Committees and is currently being managed by the University.
Figures
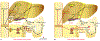
Similar articles
-
An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease.Gastroenterology. 2016 Nov;151(5):845-859. doi: 10.1053/j.gastro.2016.08.057. Epub 2016 Sep 14. Gastroenterology. 2016. PMID: 27639801 Free PMC article. Review.
-
Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction.Nat Commun. 2015 Dec 15;6:10166. doi: 10.1038/ncomms10166. Nat Commun. 2015. PMID: 26670557 Free PMC article.
-
Farnesoid X Receptor Signaling Shapes the Gut Microbiota and Controls Hepatic Lipid Metabolism.mSystems. 2016 Oct 11;1(5):e00070-16. doi: 10.1128/mSystems.00070-16. eCollection 2016 Sep-Oct. mSystems. 2016. PMID: 27822554 Free PMC article.
-
Glycine-β-muricholic acid antagonizes the intestinal farnesoid X receptor-ceramide axis and ameliorates NASH in mice.Hepatol Commun. 2022 Dec;6(12):3363-3378. doi: 10.1002/hep4.2099. Epub 2022 Oct 5. Hepatol Commun. 2022. PMID: 36196594 Free PMC article.
-
The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer.Nat Rev Gastroenterol Hepatol. 2021 May;18(5):335-347. doi: 10.1038/s41575-020-00404-2. Epub 2021 Feb 10. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33568795 Review.
Cited by
-
Capsaicin regulates lipid metabolism through modulation of bile acid/gut microbiota metabolism in high-fat-fed SD rats.Food Nutr Res. 2022 May 26;66. doi: 10.29219/fnr.v66.8289. eCollection 2022. Food Nutr Res. 2022. PMID: 35721805 Free PMC article.
-
Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity.Nutrients. 2020 Feb 25;12(3):593. doi: 10.3390/nu12030593. Nutrients. 2020. PMID: 32106420 Free PMC article.
-
Downregulation of Cyp7a1 by Cholic Acid and Chenodeoxycholic Acid in Cyp27a1/ApoE Double Knockout Mice: Differential Cardiovascular Outcome.Front Endocrinol (Lausanne). 2020 Oct 28;11:586980. doi: 10.3389/fendo.2020.586980. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193099 Free PMC article.
-
Host metabolism balances microbial regulation of bile acid signalling.Nature. 2025 Feb;638(8049):216-224. doi: 10.1038/s41586-024-08379-9. Epub 2025 Jan 8. Nature. 2025. PMID: 39779854
-
Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet.Foods. 2021 Oct 15;10(10):2457. doi: 10.3390/foods10102457. Foods. 2021. PMID: 34681506 Free PMC article.
References
-
- Seol W, Choi HS, Moore DD: Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol 1995; 9: 72–85. - PubMed
-
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C: Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995; 81: 687–693. - PubMed
-
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM: Bile acids: natural ligands for an orphan nuclear receptor. Science 1999; 284: 1365–1368. - PubMed
-
- Suh JM, Yu CT, Tang K, Tanaka T, Kodama T, Tsai MJ, Tsai SY: The expression profiles of nuclear receptors in the developing and adult kidney. Mol Endocrinol 2006; 20: 3412–3420. - PubMed
-
- Jiang T, Wang XX, Scherzer P, Wilson P, Tall-man J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M: Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 2007; 56: 2485–2493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

