Amelioration of Penetrating Ballistic-Like Brain Injury Induced Cognitive Deficits after Neuronal Differentiation of Transplanted Human Neural Stem Cells
- PMID: 28249550
- PMCID: PMC6913783
- DOI: 10.1089/neu.2016.4602
Amelioration of Penetrating Ballistic-Like Brain Injury Induced Cognitive Deficits after Neuronal Differentiation of Transplanted Human Neural Stem Cells
Abstract
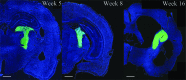
Penetrating traumatic brain injury (PTBI) is one of the major cause of death and disability worldwide. Previous studies with penetrating ballistic-like brain injury (PBBI), a PTBI rat model revealed widespread perilesional neurodegeneration, similar to that seen in humans following gunshot wound to the head, which is unmitigated by any available therapies to date. Therefore, we evaluated human neural stem cell (hNSC) engraftment to putatively exploit the potential of cell therapy that has been seen in other central nervous system injury models. Toward this objective, green fluorescent protein (GFP) labeled hNSC (400,000 per animal) were transplanted in immunosuppressed Sprague-Dawley (SD), Fisher, and athymic (ATN) PBBI rats 1 week after injury. Tacrolimus (3 mg/kg 2 days prior to transplantation, then 1 mg/kg/day), methylprednisolone (10 mg/kg on the day of transplant, 1 mg/kg/week thereafter), and mycophenolate mofetil (30 mg/kg/day) for 7 days following transplantation were used to confer immunosuppression. Engraftment in SD and ATN was comparable at 8 weeks post-transplantation. Evaluation of hNSC differentiation and distribution revealed increased neuronal differentiation of transplanted cells with time. At 16 weeks post-transplantation, neither cell proliferation nor glial lineage markers were detected. Transplanted cell morphology was similar to that of neighboring host neurons, and there was relatively little migration of cells from the peritransplant site. By 16 weeks, GFP-positive processes extended both rostrocaudally and bilaterally into parenchyma, spreading along host white matter tracts, traversing the internal capsule, and extending ∼13 mm caudally from transplantation site reaching into the brainstem. In a Morris water maze test at 8 weeks post-transplantation, animals with transplants had shorter latency to platform than vehicle-treated animals. However, weak injury-induced cognitive deficits in the control group at the delayed time point confounded benefits of durable engraftment and neuronal differentiation. Therefore, these results justify further studies to progress towards clinical translation of hNSC therapy for PTBI.
Keywords: PBBI; TBI; behavior deficit; cell transplantation; hNSC; neuronal differentiation.
Conflict of interest statement
MPH, TGH, and KKJ are employees of Neuralstem, Inc. All other authors have no competing interests to declare.
Figures






Similar articles
-
Human neural stem cell transplant location-dependent neuroprotection and motor deficit amelioration in rats with penetrating traumatic brain injury.J Trauma Acute Care Surg. 2020 Apr;88(4):477-485. doi: 10.1097/TA.0000000000002510. J Trauma Acute Care Surg. 2020. PMID: 31626023 Free PMC article.
-
Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury.Exp Neurol. 2016 Jul;281:1-16. doi: 10.1016/j.expneurol.2016.04.008. Epub 2016 Apr 11. Exp Neurol. 2016. PMID: 27079998
-
Assessing fetal human neural stem cells tumorigenicity potential in athymic rats with penetrating traumatic brain injury (pTBI).Brain Res. 2022 Sep 15;1791:148002. doi: 10.1016/j.brainres.2022.148002. Epub 2022 Jul 8. Brain Res. 2022. PMID: 35810769 Clinical Trial.
-
Circuit integration by transplanted human neurons.Curr Opin Genet Dev. 2024 Dec;89:102225. doi: 10.1016/j.gde.2024.102225. Curr Opin Genet Dev. 2024. PMID: 39586651 Review.
-
Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation.Dis Model Mech. 2009 Jan-Feb;2(1-2):23-38. doi: 10.1242/dmm.001198. Dis Model Mech. 2009. PMID: 19132123 Free PMC article. Review.
Cited by
-
Enduring Neuroprotective Effect of Subacute Neural Stem Cell Transplantation After Penetrating TBI.Front Neurol. 2019 Jan 17;9:1097. doi: 10.3389/fneur.2018.01097. eCollection 2018. Front Neurol. 2019. PMID: 30719019 Free PMC article. Review.
-
Stem cells technology: a powerful tool behind new brain treatments.Drug Deliv Transl Res. 2018 Oct;8(5):1564-1591. doi: 10.1007/s13346-018-0548-y. Drug Deliv Transl Res. 2018. PMID: 29916013 Review.
-
Human neural stem cell transplant location-dependent neuroprotection and motor deficit amelioration in rats with penetrating traumatic brain injury.J Trauma Acute Care Surg. 2020 Apr;88(4):477-485. doi: 10.1097/TA.0000000000002510. J Trauma Acute Care Surg. 2020. PMID: 31626023 Free PMC article.
-
Human neural stem cell transplants to address multiple pathologies associated with traumatic brain injury.Neural Regen Res. 2019 Oct;14(10):1699-1700. doi: 10.4103/1673-5374.255620. Neural Regen Res. 2019. PMID: 31169178 Free PMC article. No abstract available.
-
Open-Field Blast Injury Disrupts Corneal Gene Expression Linked to Ion Transport, Sensory Perception, and Neural Signaling.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):68. doi: 10.1167/iovs.66.11.68. Invest Ophthalmol Vis Sci. 2025. PMID: 40862667 Free PMC article.
References
-
- Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 - PubMed
-
- Gold E.M., Su D., Lopez–Velazquez L., Haus D.L., Perez H., Lacuesta G.A., Anderson A.J., and Cummings B.J. (2013). Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen. Med. 8, 483–516 - PubMed
-
- Tasigiorgos S., Economopoulos K.P., Winfield R.D., and Sakran J.V. (2015). Firearm injury in the United States: an overview of an evolving public health problem. J. Am. Coll. Surg. 221, 1005–1014 - PubMed
-
- Joseph B., Aziz H., Pandit V., Kulvatunyou N., O'Keeffe T., Wynne J., Tang A., Friese R.S., and Rhee P. (2014). Improving survival rates after civilian gunshot wounds to the brain. J. Am. Coll. Surg. 218, 58–65 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

