Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature
- PMID: 28249574
- PMCID: PMC5333431
- DOI: 10.1186/s12879-017-2287-y
Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature
Abstract
Background: Reactivation of occult or inactive Hepatitis B virus (HBV) infection during immunosuppressant treatments is well known and widely described in literature. The same observation has been made in Hepatitis C (HCV)-infected patients previously exposed to HBV and treated with interferon-free DAA treatments. Because of common transmission routes, persons may have been exposed to HCV, HBV and HIV, but few cases have been reported in this scenario to date. Frequency of HBV reactivation in HIV/HCV co-infected patients previously exposed to HBV and treated with DAA remains unclear. Herein, we report an episode of HBV reactivation in an HIV/HCV co-infected patient prescribed with sofosbuvir/ledipasvir for HCV.
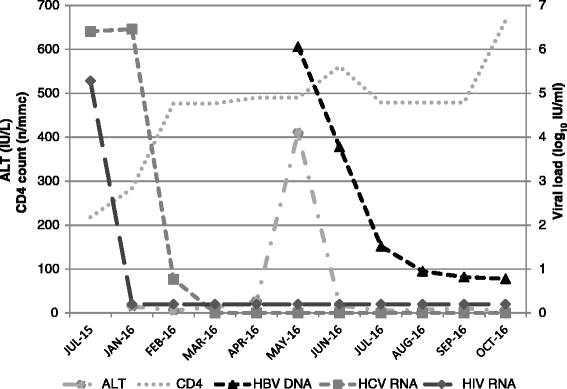
Case presentation: The patient is a Caucasian 54-years old female, with HIV/HCV co-infection (genotype 4), and a previous exposure to HBV, documented by negativity of HBsAg and positivity of HBsAb and HBcAb. Her medical history included: myocardial infarct, chronic kidney disease stage 3, chronic obstructive pulmonary disease, and mild pulmonary hypertension. HCV had not been treated with interferon (IFN)-based regimens and liver stiffness was 10.5 KPa (Metavir stage F3) at hepatic elastography. Because of CKD, she was prescribed with a nucleoside reverse transcriptase (NRTI)-sparing regimen including darunavir/ritonavir plus etravirine, and thereafter with sofosbuvir/ledipasvir for 12 weeks. Four weeks after DAA termination, the patient was hospitalized with symptoms of acute hepatitis. Blood tests showed HCV RNA <12 IU/ml, but positivity of HBAg, HBeAg, and of anti-core antibodies (IgM and IgG), while anti-HBs and anti-HBe antibodies were negative. HBV DNA was 6.06 Log10 IU/ml. Entecavir was started obtaining resolution of symptoms, normalization of liver enzymes, as well as reduction of HBV DNA and of quantitative HBV surface antigen.
Conclusions: This case-report highlights the risk of HBV reactivation with interferon-free DAA treatment in HIV/HCV co-infected patients previously exposed to HBV and who have contraindications for treatment with nucleoside/nucleotide reverse transcriptase Inhibitors because of comorbid conditions. In the setting of HIV infection, clinicians prescribing DAA should be aware of this risk, and HBV assessment at treatment start as well as virological monitoring during DAA treatment is recommended. Large epidemiological and virological studies are needed to investigate reactivation of occult HBV infection more in depth.
Keywords: Acute hepatitis; DAA; HBV reactivation; HIV; Sofosbuvir/ledipasvir.
Figures
References
-
- Potthoff A, Berg T, Wedemeyer H, HEP-NET B/C Coinfection Study Group Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol. 2009;44:1487–90. doi: 10.3109/00365520903329585. - DOI - PubMed
-
- Soriano V. Reactivation of Hepatitis B in HIV Patients Treated for Hepatitis C. AIDS Rev. 2016;18:222–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous