Parathyroid hormone's enhancement of bones' osteogenic response to loading is affected by ageing in a dose- and time-dependent manner
- PMID: 28249797
- PMCID: PMC5404907
- DOI: 10.1016/j.bone.2017.02.009
Parathyroid hormone's enhancement of bones' osteogenic response to loading is affected by ageing in a dose- and time-dependent manner
Abstract
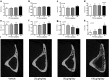
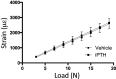
Decreased effectiveness of bones' adaptive response to mechanical loading contributes to age-related bone loss. In young mice, intermittent administration of parathyroid hormone (iPTH) at 20-80μg/kg/day interacts synergistically with artificially applied loading to increase bone mass. Here we report investigations on the effect of different doses and duration of iPTH treatment on mice whose osteogenic response to artificial loading is impaired by age. One group of aged, 19-month-old female C57BL/6 mice was given 0, 25, 50 or 100μg/kg/day iPTH for 4weeks. Histological and μCT analysis of their tibiae revealed potent iPTH dose-related increases in periosteally-enclosed area, cortical area and porosity with decreased cortical thickness. There was practically no effect on trabecular bone. Another group was given a submaximal dose of 50μg/kg/day iPTH or vehicle for 2 or 6weeks with loading of their right tibia three times per week for the final 2weeks. In the trabecular bone of these mice the loading-related increase in BV/TV was abrogated by iPTH primarily by reduction of the increase in trabecular number. In their cortical bone, iPTH treatment time-dependently increased cortical porosity. Loading partially reduced this effect. The osteogenic effects of iPTH and loading on periosteally-enclosed area and cortical area were additive but not synergistic. Thus in aged, unlike young mice, iPTH and loading appear to have separate effects. iPTH alone causes a marked increase in cortical porosity which loading reduces. Both iPTH and loading have positive effects on cortical periosteal bone formation but these are additive rather than synergistic.
Keywords: Ageing; Bone; Mechanical loading; Osteoporosis; Parathyroid hormone.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice.Bone. 2008 Aug;43(2):238-248. doi: 10.1016/j.bone.2008.04.012. Epub 2008 May 1. Bone. 2008. PMID: 18539556
-
The cyclooxygenase-2 selective inhibitor NS-398 does not influence trabecular or cortical bone gain resulting from repeated mechanical loading in female mice.Osteoporos Int. 2013 Jan;24(1):383-8. doi: 10.1007/s00198-012-1922-0. Epub 2012 Feb 14. Osteoporos Int. 2013. PMID: 22349912 Free PMC article.
-
Disuse rescues the age-impaired adaptive response to external loading in mice.Osteoporos Int. 2015 Nov;26(11):2703-8. doi: 10.1007/s00198-015-3142-x. Epub 2015 Apr 29. Osteoporos Int. 2015. PMID: 25920749 Free PMC article.
-
Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure.Bone. 2007 Jun;40(6):1447-52. doi: 10.1016/j.bone.2006.09.008. Epub 2006 Oct 12. Bone. 2007. PMID: 17045858 Review.
-
[Parathyroid and bone. Effect of parathyroid hormone on bone quality].Clin Calcium. 2007 Dec;17(12):1858-64. Clin Calcium. 2007. PMID: 18057661 Review. Japanese.
Cited by
-
Teriparatide and Abaloparatide Have a Similar Effect on Bone in Mice.Front Endocrinol (Lausanne). 2021 Apr 19;12:628994. doi: 10.3389/fendo.2021.628994. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33953694 Free PMC article.
-
Stress and Alterations in Bones: An Interdisciplinary Perspective.Front Endocrinol (Lausanne). 2017 May 1;8:96. doi: 10.3389/fendo.2017.00096. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28507534 Free PMC article. Review.
-
Methodological aspects of in vivo axial loading in rodents: a systematic review.J Musculoskelet Neuronal Interact. 2023 Jun 1;23(2):236-262. J Musculoskelet Neuronal Interact. 2023. PMID: 37259664 Free PMC article.
-
Humble Bones. From skeletogenesis to the Utah paradigm of skeletal physiology. A tribute to the memories of Webster S.S. Jee and Harold M. Frost.J Musculoskelet Neuronal Interact. 2018 Sep 1;18(3):281-282. J Musculoskelet Neuronal Interact. 2018. PMID: 30179203 Free PMC article. No abstract available.
-
Intermittent Parathyroid Hormone Accelerates Stress Fracture Healing More Effectively Following Cessation of Bisphosphonate Treatment.JBMR Plus. 2020 Aug 6;4(9):e10387. doi: 10.1002/jbm4.10387. eCollection 2020 Sep. JBMR Plus. 2020. PMID: 32995690 Free PMC article.
References
-
- Frost H.M. ISMNI; 1960. The Utah Paradigm of Skeletal Physiology. - PubMed
-
- Willie B.M., Birkhold A.I., Razi H., Thiele T., Aido M., Kruck B. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55:335–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

