LIGHT Elevation Enhances Immune Eradication of Colon Cancer Metastases
- PMID: 28249900
- PMCID: PMC5410174
- DOI: 10.1158/0008-5472.CAN-16-1655
LIGHT Elevation Enhances Immune Eradication of Colon Cancer Metastases
Abstract
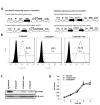
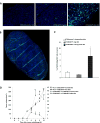
The majority of patients with colon cancer will develop advanced disease, with the liver being the most common site of metastatic disease. Patients with increased numbers of tumor-infiltrating lymphocytes in primary colon tumors and liver metastases have improved outcomes. However, the molecular factors that could empower antitumor immune responses in this setting remain to be elucidated. We reported that the immunostimulatory cytokine LIGHT (TNFSF14) in the microenvironment of colon cancer metastases associates with improved patient survival, and here we demonstrate in an immunocompetent murine model that colon tumors expressing LIGHT stimulate lymphocyte proliferation and tumor cell-specific antitumor immune responses. In this model, increasing LIGHT expression in the microenvironment of either primary tumors or liver metastases triggered regression of established tumors and slowed the growth of liver metastases, driven by cytotoxic T-lymphocyte-mediated antitumor immunity. These responses corresponded with significant increases in tumor-infiltrating lymphocytes and increased expression of lymphocyte-homing signals in the metastatic tumors. Furthermore, we demonstrated evidence of durable tumor-specific antitumor immunity. In conclusion, increasing LIGHT expression increased T-cell proliferation, activation, and infiltration, resulting in enhanced tumor-specific immune-mediated tumor regressions in primary tumors and colorectal liver metastases. Mechanisms to increase LIGHT in the colon cancer microenvironment warrant further investigation and hold promise as an immunotherapeutic strategy. Cancer Res; 77(8); 1880-91. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The authors have declared that no conflict to interest exists
Figures



Similar articles
-
Combination Immunotherapy With LIGHT and Interleukin-2 Increases CD8 Central Memory T-Cells In Vivo.J Surg Res. 2021 Jul;263:44-52. doi: 10.1016/j.jss.2021.01.010. Epub 2021 Feb 22. J Surg Res. 2021. PMID: 33631377 Free PMC article.
-
Genetic evidence that intratumoral T-cell proliferation and activation are associated with recurrence and survival in patients with resected colorectal liver metastases.Cancer Immunol Res. 2015 Apr;3(4):380-8. doi: 10.1158/2326-6066.CIR-14-0212. Epub 2015 Jan 19. Cancer Immunol Res. 2015. PMID: 25600439 Free PMC article.
-
Shedding LIGHT (TNFSF14) on the tumor microenvironment of colorectal cancer liver metastases.J Transl Med. 2013 Mar 20;11:70. doi: 10.1186/1479-5876-11-70. J Transl Med. 2013. PMID: 23514280 Free PMC article.
-
Targeting tumors with LIGHT to generate metastasis-clearing immunity.Cytokine Growth Factor Rev. 2008 Jun-Aug;19(3-4):285-94. doi: 10.1016/j.cytogfr.2008.04.004. Epub 2008 May 27. Cytokine Growth Factor Rev. 2008. PMID: 18508404 Free PMC article. Review.
-
TNFSF14: LIGHTing the Way for Effective Cancer Immunotherapy.Front Immunol. 2020 May 15;11:922. doi: 10.3389/fimmu.2020.00922. eCollection 2020. Front Immunol. 2020. PMID: 32499782 Free PMC article. Review.
Cited by
-
The Prognostic Landscape of Tumor-Infiltrating Immune Cells and Immune Checkpoints in Glioblastoma.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819869949. doi: 10.1177/1533033819869949. Technol Cancer Res Treat. 2019. PMID: 31451090 Free PMC article.
-
Hepatic stellate cell mediates transcription of TNFSF14 in hepatocellular carcinoma cells via H2S/CSE-JNK/JunB signaling pathway.Cell Death Dis. 2022 Mar 15;13(3):238. doi: 10.1038/s41419-022-04678-z. Cell Death Dis. 2022. Retraction in: Cell Death Dis. 2022 Nov 30;13(12):1012. doi: 10.1038/s41419-022-05464-7. PMID: 35292636 Free PMC article. Retracted.
-
TNFSF14-Derived Molecules as a Novel Treatment for Obesity and Type 2 Diabetes.Int J Mol Sci. 2021 Sep 30;22(19):10647. doi: 10.3390/ijms221910647. Int J Mol Sci. 2021. PMID: 34638990 Free PMC article.
-
Biomaterial-Based Activation and Expansion of Tumor-Specific T Cells.Front Immunol. 2019 May 3;10:931. doi: 10.3389/fimmu.2019.00931. eCollection 2019. Front Immunol. 2019. PMID: 31130945 Free PMC article. Review.
-
TNFSF14/LIGHT, a Non-Canonical NF-κB Stimulus, Induces the HIF Pathway.Cells. 2018 Aug 8;7(8):102. doi: 10.3390/cells7080102. Cells. 2018. PMID: 30096845 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. - PubMed
-
- Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. Journal of Clinical Oncology. 2005;23(9):2038–48. - PubMed
-
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25(29):4575–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

