Endothelin Promotes Colorectal Tumorigenesis by Activating YAP/TAZ
- PMID: 28249901
- PMCID: PMC6724531
- DOI: 10.1158/0008-5472.CAN-16-3229
Endothelin Promotes Colorectal Tumorigenesis by Activating YAP/TAZ
Abstract
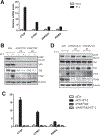
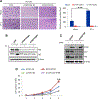
Endothelin receptor A (ETAR) promotes tumorigenesis by stimulating cell proliferation, migration, and survival. However, the mechanism of ETAR in promoting tumor growth is largely unknown. In this study, we demonstrate that ETAR stimulates colon cell proliferation, migration, and tumorigenesis through the activation of YAP/TAZ, two transcription coactivators of the Hippo tumor suppressor pathway. Endothelin-1 treatment induced YAP/TAZ dephosphorylation, nuclear accumulation, and transcriptional activation in multiple colon cancer cells. ETAR stimulation acted via downstream G-protein Gαq/11 and Rho GTPase to suppress the Hippo pathway, thus leading to YAP/TAZ activation, which was required for ETAR-induced tumorigenesis. Overall, these results indicate a critical role of the YAP/TAZ axis in ETAR signaling. Cancer Res; 77(9); 2413-23. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
K.-L. Guanhas ownership interest (includingpatents) inVivace. No potential conflicts of interest were disclosed by the other authors.
Figures




References
-
- Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol 2008;86:485–98. - PubMed
-
- Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000;102:2434–40. - PubMed
-
- Wu MH, Chen LM, Hsu HH, Lin JA, Lin YM, Tsai FJ, et al. Endothelin-1 enhances cell migration through COX-2 up-regulation in human chondrosarcoma. Biochim Biophys Acta 2013;1830:3355–64. - PubMed
-
- Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogen- esis. Trends Endocrinol Metab 2003;14:44–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

