Population pharmacokinetics of intravenous Erwinia asparaginase in pediatric acute lymphoblastic leukemia patients
- PMID: 28250007
- PMCID: PMC5394946
- DOI: 10.3324/haematol.2016.149195
Population pharmacokinetics of intravenous Erwinia asparaginase in pediatric acute lymphoblastic leukemia patients
Abstract
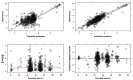
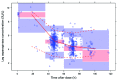
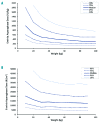
Erwinia asparaginase is an important component in the treatment of pediatric acute lymphoblastic leukemia. A large variability in serum concentrations has been observed after intravenous Erwinia asparaginase. Currently, Dutch Childhood Oncology Group protocols dose alterations are based on trough concentrations to ensure adequate asparaginase activity (≥100 IU/L). The aim of this study was to describe the population pharmacokinetics of intravenous Erwinia asparaginase to quantify and gather insight into inter-individual and inter-occasion variability. The starting dose was evaluated on the basis of the derived population pharmacokinetic parameters. In a multicenter prospective observational study, a total of 714 blood samples were collected from 51 children (age 1-17 years) with acute lymphoblastic leukemia. The starting dose was 20,000 IU/m2 three times a week and adjusted according to trough levels from week three onwards. A population pharmacokinetic model was developed using NONMEM® A 2-compartment linear model with allometric scaling best described the data. Inter-individual and inter-occasion variability of clearance were 33% and 13%, respectively. Clearance in the first month of treatment was 14% higher (P<0.01). Monte Carlo simulations with our pharmacokinetic model demonstrated that patients with a low weight might require higher doses to achieve similar concentrations compared to patients with high weight. The current starting dose of 20,000 IU/m2 might result in inadequate concentrations, especially for smaller, lower weight patients, hence dose adjustments based on individual clearance are recommended. The protocols were approved by the institutional review boards. (Registered at NTR 3379 Dutch Trial Register; www.trialregister.nl).
Copyright© Ferrata Storti Foundation.
Figures



References
-
- Dubbers A, Wurthwein G, Muller HJ, et al. Asparagine synthetase activity in paediatric acute leukaemias: AML-M5 subtype shows lowest activity. Br J Haematol. 2000; 109(2):427–429. - PubMed
-
- Capizzi RL, Bertino JR, Skeel RT, et al. Clinical, Biochemical, Pharmacological, and Immunological Studies. Ann Intern Med. 1971;74(6):893–901. - PubMed
-
- Jaffe N, Traggis D, Das L, et al. L-asparaginase in the treatment of neoplastic diseases in children. Cancer Res. 1971;31(7):942–949. - PubMed
-
- Pession A. Long-Term Results of a Randomized Trial on Extended Use of High Dose L-Asparaginase for Standard Risk Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2005;23(28):7161–7167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

