Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists
- PMID: 28250025
- PMCID: PMC5408601
- DOI: 10.1194/jlr.D073148
Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists
Abstract
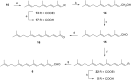
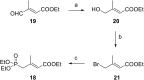
Consumption of the tomato carotenoid, lycopene, has been associated with favorable health benefits. Some of lycopene's biological activity may be due to metabolites resulting from cleavage of the lycopene molecule. Because of their structural similarity to the retinoic acid receptor (RAR) antagonist, β-apo-13-carotenone, the "first half" putative oxidative cleavage products of the symmetrical lycopene have been synthesized. All transformations proceed in moderate to good yield and some with high stereochemical integrity allowing ready access to these otherwise difficult to obtain terpenoids. In particular, the methods described allow ready access to the trans isomers of citral (geranial) and pseudoionone, important flavor and fragrance compounds that are not readily available isomerically pure and are building blocks for many of the longer apolycopenoids. In addition, all of the apo-11, apo-13, and apo-15 lycopenals/lycopenones/lycopenoic acids have been prepared. These compounds have been evaluated for their effect on RAR-induced genes in cultured hepatoma cells and, much like β-apo-13-carotenone, the comparable apo-13-lycopenone and the apo-15-lycopenal behave as RAR antagonists. Furthermore, molecular modeling studies demonstrate that the apo-13-lycopenone efficiently docked into the ligand binding site of RARα. Finally, isothermal titration calorimetry studies reveal that apo-13-lycopenone acts as an antagonist of RAR by inhibiting coactivator recruitment to the receptor.
Keywords: apolipoprotein-13-lycopenone; apolycopenoids; chemical synthesis; diet and dietary lipids; gene expression; nuclear receptors; retinoic acid receptor antagonist; retinoids/vitamin A.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


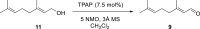



References
-
- Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W. Jr., and Harrison E. H.. 2012. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287: 15886–15895. - PMC - PubMed
-
- Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., and Blaner W. S.. 2001. Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J. Biol. Chem. 276: 32160–32168. - PubMed
-
- Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., and Gronemeyer H.. 2006. International union of pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58: 712–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

