A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness
- PMID: 28250420
- PMCID: PMC5446789
- DOI: 10.1038/nmat4874
A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness
Abstract
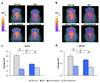
The degeneration of photoreceptors in the retina is one of the major causes of adult blindness in humans. Unfortunately, no effective clinical treatments exist for the majority of retinal degenerative disorders. Here we report on the fabrication and functional validation of a fully organic prosthesis for long-term in vivo subretinal implantation in the eye of Royal College of Surgeons rats, a widely recognized model of retinitis pigmentosa. Electrophysiological and behavioural analyses reveal a prosthesis-dependent recovery of light sensitivity and visual acuity that persists up to 6-10 months after surgery. The rescue of the visual function is accompanied by an increase in the basal metabolic activity of the primary visual cortex, as demonstrated by positron emission tomography imaging. Our results highlight the possibility of developing a new generation of fully organic, highly biocompatible and functionally autonomous photovoltaic prostheses for subretinal implants to treat degenerative blindness.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. - PubMed
-
- Smith AJ, Bainbridge JW, Ali RR. Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther. 2012;19:154–161. - PubMed
-
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. - PubMed
-
- Frasson M, et al. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med. 1999;5:1183–1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

