PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting
- PMID: 28250726
- PMCID: PMC5327624
- DOI: 10.5114/pm.2016.65667
PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting
Abstract
Poly(ADP-ribose) polymerases have shown true promise in early clinical studies due to reported activity in BRCA-associated cancers. PARP inhibitors may represent a potentially important new class of chemotherapeutic agents directed at targeting cancers with defective DNA-damage repair. In order to widen the prospective patient population that would benefit from PARP inhibitors, predictive biomarkers based on a clear understanding of the mechanism of action are required. In addition, a more sophisticated understanding of the toxicity profile is required if PARP inhibitors are to be employed in the curative, rather than the palliative, setting. PARP inhibitors have successfully moved into clinical practice in the past few years, with approval granted from the Food and Drug Administration (FDA) and European Medicines Agency (EMA) within the past two years. The United States FDA approval of olaparib applies to fourth-line treatment in germline BRCA-mutant ovarian cancer, and European EMA approval of olaparib for maintenance therapy in both germline and somatic BRCA-mutant platinum-sensitive ovarian cancer. This review covers the current understanding of PARP, its inhibition, and the basis of the excitement surrounding these new agents. It also evaluates future approaches and directions required to achieve full understanding of the intricate interplay of these agents at the cellular level.
Keywords: BRCA; DNA; PARP; PARP1; PARP2; mutation; olaparib; ovarian cancer; poly(ADP-ribose) polymerases.
Figures
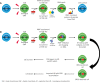
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Buys SS, Partridge E, Black A, et al. for the PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. - PubMed
-
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2011, National Cancer Institute [Internet] Bethesda: National Cancer Institute; 2014.
-
- Porter PL. Global trends in breast cancer incidence and mortality. Salud Pública de México. 2009;51(suppl 2):s141–146. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
