Capacity for ethical and regulatory review of herbal trials in developing countries: a case study of Moringa oleifera research in HIV-infected patients
- PMID: 28250958
- PMCID: PMC5322785
- DOI: 10.1186/s40545-017-0099-5
Capacity for ethical and regulatory review of herbal trials in developing countries: a case study of Moringa oleifera research in HIV-infected patients
Abstract
Background: Lack of regulatory capacity limits the conduct of ethical and rigorous trials of herbal medicines in developing countries. Sharing ethical and regulatory experiences of successful herbal trials may accelerate the field while assuring human subjects protection. The methods and timelines for the ethical and regulatory review processes for the first drug regulatory authority approved herbal trial in Zimbabwe are described in this report.
Methods: The national drug regulatory authority and ethics committee were engaged for pre-submission discussions. Six applications were submitted. Application procedures and communications with the various regulatory and ethics review boards were reviewed. Key issues raised and timelines for communications were summarized.
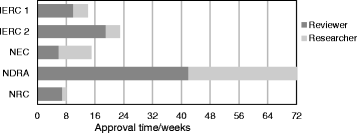
Results: There was no special framework for the approval of herbal trials. One local institutional review committee granted an exemption. Key issues raised for revision were around pre-clinical efficacy and safety data, standardization and quality assurance of the intervention as well as consenting procedures. Approval timelines ranged between eight and 72 weeks.
Conclusions: In the absence of a defined framework for review of herbal trials, approval processes can be delayed. Dialogue between researchers and regulators is important for successful and efficient protocol approval for herbal trials in developing countries.
Trial registration: The study was registered prospectively on August 3, 2011 with clinicaltrials.gov (NCT01410058).
Keywords: Developing countries; Herbal trial; Regulation.
Figures
References
-
- Koonrungsesomboon N and Karbwang J. Ethical considerations in clinical research on herbal medicine for prevention of cardiovascular disease in the ageing. Phytomed. 2015;doi: 10.1016/j.phymed.2015.10.017. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical