MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells
- PMID: 28250973
- PMCID: PMC5327502
- DOI: 10.1038/cddiscovery.2017.13
MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells
Abstract
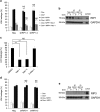
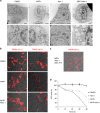
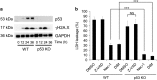
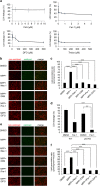
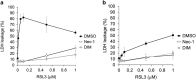
Regulation of cell death is potentially a powerful treatment modality for intractable diseases such as neurodegenerative diseases. Although there have been many reports about the possible involvement of various types of cell death in neurodegenerative diseases, it is still unclear exactly how neurons die in patients with these diseases, thus treatment strategies based on cell death regulation have not been established yet. To obtain some insight into the mechanisms of cell death involved in neurodegenerative diseases, we studied the effect of 1-methyl-4-phenylpyridinium (MPP+) on the human neuroblastoma cell line SH-SY5Y (a widely used model of Parkinson's disease). We found that MPP+ predominantly induced non-apoptotic death of neuronally differentiated SH-SY5Y cells. This cell death was strongly inhibited by necrostatin-1 (Nec-1), a necroptosis inhibitor, and by an indole-containing compound (3,3'-diindolylmethane: DIM). However, it occurred independently of receptor-interacting serine/threonine-protein kinase 1/3 (RIP1/RIP3), indicating that this form of cell death was not necroptosis. MPP+-induced cell death was also inhibited by several inhibitors of ferroptosis, including ferrostatin-1 (Fer-1). Although MPP+-induced death and ferroptosis shared some features, such as occurrence of lipid peroxidation and inhibition by Fer-1, MPP+-induced death seemed to be distinct from ferroptosis because MPP+-induced death (but not ferroptosis) was inhibited by Nec-1, was independent of p53, and was accompanied by ATP depletion and mitochondrial swelling. Further investigation of MPP+-induced non-apoptotic cell death may be useful for understanding the mechanisms of neuronal loss and for treatment of neurodegenerative diseases such as Parkinson's disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 2003; 348: 1365–1375. - PubMed
-
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 2000; 1: 120–130. - PubMed
-
- Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 2003; 4: 365–375. - PubMed
-
- Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE 2006; 2006: pe44. - PubMed
-
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6: 1221–1228. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

