Serum Proteome Alterations in Patients with Cognitive Impairment after Traumatic Brain Injury Revealed by iTRAQ-Based Quantitative Proteomics
- PMID: 28251161
- PMCID: PMC5303854
- DOI: 10.1155/2017/8572509
Serum Proteome Alterations in Patients with Cognitive Impairment after Traumatic Brain Injury Revealed by iTRAQ-Based Quantitative Proteomics
Abstract
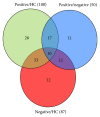
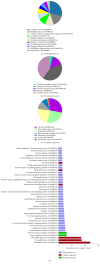
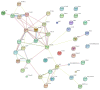
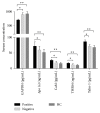
Background. Cognitive impairment is the leading cause of traumatic brain injury- (TBI-) related disability; however, the underlying pathogenesis of this dysfunction is not completely understood. Methods. Using an isobaric tagging for relative and absolute quantitation- (iTRAQ-) based quantitative proteomic approach, serum samples from healthy control subjects, TBI patients with cognitive impairment, and TBI patients without cognitive impairment were analysed to identify differentially expressed proteins (DEPs) related to post-TBI cognitive impairment. In addition, DEPs were further analysed using bioinformatic platforms and validated using enzyme-linked immunosorbent assays (ELISA). Results. A total of 56 DEPs were identified that were specifically related to TBI-induced cognitive impairment. Bioinformatic analysis revealed that a wide variety of cellular and metabolic processes and some signaling pathways were involved in the pathophysiology of cognitive deficits following TBI. Five randomly selected DEPs were validated using ELISA in an additional 105 cases, and the results also supported the experimental findings. Conclusions. Despite limitations, our findings will facilitate further studies of the pathological mechanisms underlying TBI-induced cognitive impairment and provide new methods for the research and development of neuroprotective agents. However, further investigation on a large cohort is warranted.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
iTRAQ-based proteomic profiling reveals protein alterations after traumatic brain injury and supports thyroxine as a potential treatment.Mol Brain. 2021 Jan 27;14(1):25. doi: 10.1186/s13041-021-00739-0. Mol Brain. 2021. PMID: 33504361 Free PMC article.
-
Serum quantitative proteomic analysis of patients with keshan disease based on iTRAQ labeling technique: A first term study.J Trace Elem Med Biol. 2017 Dec;44:331-338. doi: 10.1016/j.jtemb.2017.09.012. Epub 2017 Sep 18. J Trace Elem Med Biol. 2017. PMID: 28965596
-
Complement cascade on severe traumatic brain injury patients at the chronic unconscious stage: implication for pathogenesis.Expert Rev Mol Diagn. 2018 Aug;18(8):761-766. doi: 10.1080/14737159.2018.1471985. Epub 2018 May 14. Expert Rev Mol Diagn. 2018. PMID: 29718755
-
Cognitive impairment after traumatic brain injury: The role of MRI and possible pathological basis.J Neurol Sci. 2016 Nov 15;370:244-250. doi: 10.1016/j.jns.2016.09.049. Epub 2016 Sep 25. J Neurol Sci. 2016. PMID: 27772768 Review.
-
Amantadine to Treat Cognitive Dysfunction in Moderate to Severe Traumatic Brain Injury.J Trauma Nurs. 2015 Jul-Aug;22(4):194-203; quiz E1-2. doi: 10.1097/JTN.0000000000000138. J Trauma Nurs. 2015. PMID: 26165872 Review.
Cited by
-
Identification of perioperative neurocognitive dysfunction biomarkers in cerebrospinal fluid with quantitative proteomic approach in patients undergoing transurethral resection of prostate with combined spinal and epidural analgesia.Medicine (Baltimore). 2022 Sep 9;101(36):e30448. doi: 10.1097/MD.0000000000030448. Medicine (Baltimore). 2022. PMID: 36086739 Free PMC article.
-
iTRAQ-Based Proteomics Analysis Reveals the Effect of Rhubarb in Rats with Ischemic Stroke.Biomed Res Int. 2018 Jul 16;2018:6920213. doi: 10.1155/2018/6920213. eCollection 2018. Biomed Res Int. 2018. PMID: 30112417 Free PMC article.
-
Single-cell RNA and transcriptome sequencing profiles identify immune-associated key genes in the development of diabetic kidney disease.Front Immunol. 2023 Mar 29;14:1030198. doi: 10.3389/fimmu.2023.1030198. eCollection 2023. Front Immunol. 2023. PMID: 37063851 Free PMC article.
-
Quantitative proteomic characterization of microvesicles/exosomes from the cerebrospinal fluid of patients with acute bilirubin encephalopathy.Mol Med Rep. 2020 Aug;22(2):1257-1268. doi: 10.3892/mmr.2020.11194. Epub 2020 May 28. Mol Med Rep. 2020. PMID: 32468033 Free PMC article.
-
Synovial tissue quantitative proteomics analysis reveals paeoniflorin decreases LIFR and ASPN proteins in experimental rheumatoid arthritis.Drug Des Devel Ther. 2018 Mar 6;12:463-473. doi: 10.2147/DDDT.S153927. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29551890 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

