Quality assurance guidelines for superficial hyperthermia clinical trials : II. Technical requirements for heating devices
- PMID: 28251250
- PMCID: PMC5405104
- DOI: 10.1007/s00066-017-1106-0
Quality assurance guidelines for superficial hyperthermia clinical trials : II. Technical requirements for heating devices
Abstract
Quality assurance (QA) guidelines are essential to provide uniform execution of clinical trials with uniform quality hyperthermia treatments. This document outlines the requirements for appropriate QA of all current superficial heating equipment including electromagnetic (radiative and capacitive), ultrasound, and infrared heating techniques. Detailed instructions are provided how to characterize and document the performance of these hyperthermia applicators in order to apply reproducible hyperthermia treatments of uniform high quality. Earlier documents used specific absorption rate (SAR) to define and characterize applicator performance. In these QA guidelines, temperature rise is the leading parameter for characterization of applicator performance. The intention of this approach is that characterization can be achieved with affordable equipment and easy-to-implement procedures. These characteristics are essential to establish for each individual applicator the specific maximum size and depth of tumors that can be heated adequately. The guidelines in this document are supplemented with a second set of guidelines focusing on the clinical application. Both sets of guidelines were developed by the European Society for Hyperthermic Oncology (ESHO) Technical Committee with participation of senior Society of Thermal Medicine (STM) members and members of the Atzelsberg Circle.
Um eine einheitliche Durchführung klinischer Studien in der Hyperthermie zu gewährleisten, sind Leitlinien zur Qualitätssicherung (QA) unerlässlich. Dieses Dokument enthält die Anforderungen zur QA für alle aktuellen Therapiegeräte zur lokalen Hyperthermie, inklusive elektromagnetischer Systeme (radiativ und kapazitiv), Ultraschall- und Infrarottechnik. In detaillierten Anleitungen wird erklärt, wie die Leistungscharakteristik der Hyperthermieapplikatoren zu beschreiben und zu dokumentieren ist, um reproduzierbare, einheitliche und qualitativ hochwertige Hyperthermiebehandlungen zu gewährleisten. Im Gegensatz zu früheren Leitlinien wird anstelle der spezifischen Absorptionsrate (SAR) jetzt der Temperaturanstieg als maßgeblicher Parameter für die Beschreibung der Leistungscharakteristik von Hyperthermieapplikatoren verwendet. Grund für diesen Ansatz ist eine möglichst einfache und kostengünstige Leistungsbeschreibung. Diese ist notwendig, um für jeden einzelnen Applikator die maximale Tumorgröße und -tiefe zu bestimmen, die adäquat überwärmt werden kann. Dieses Dokument wird durch die Leitlinie zur klinischen Durchführung der lokalen Hyperthermie vervollständigt. Beide Teile der Leitlinie wurden vom Technischen Komitee der European Society for Hyperthermic Oncology (ESHO) in Zusammenarbeit mit leitenden Mitgliedern der Society of Thermal Medicine (STM) und Mitgliedern des Atzelsberger Kreises erarbeitet.
Keywords: Applicator; Heating criteria; Hyperthermia, superficial; Phantoms; Quality assurance; Water bolus.
Conflict of interest statement
Conflict of interest
C. G. Van Rhoon: ESHO president; Advisor Pyrexar Medical Inc and Sensius; Member Health Council of the Netherlands; Shares: Sensius. H. Dobšíček Trefná, J. Crezee, M. Schmidt, D. Marder, U. Lamprecht, M. Ehmann, J. Nadobny, J. Hartmann, N. Lomax, S. Abdel-Rahman, S. Curto Ramos, A. Bakker, M.D. Hurwitz, C.J. Diederich, and P.R. Stauffer declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
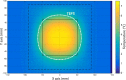

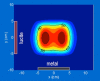
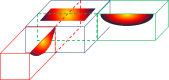
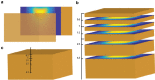
References
-
- Atzelsberg Circle for Clinical Hyperthermia, http://atzelsbergerkreis.de. Last access: 24.02.2017
-
- ESHO, www.esho.info. Last access: 24.02.2017
-
- STM, http://www.thermaltherapy.org/eBusSFTM/. Last access: 24.02.2017
-
- Bruggmoser G, Atzelsberg Research Group. European Society for Hyperthermic Oncology - Guideline for the clinical application documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol. 2012;188(Suppl 2):198–211. doi: 10.1007/s00066-012-0176-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

