Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA
- PMID: 28251792
- PMCID: PMC5573947
- DOI: 10.1111/dom.12927
Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA
Abstract
Aims: To evaluate short- and long-term glycaemic control and hypoglycaemia incidence in insulin-naïve patients ≥30 years of age with type 2 diabetes (T2DM) initiating basal insulin (BI) with or without oral anti-hyperglycaemic drugs (OADs).
Methods: This was an observational, retrospective longitudinal analysis of electronic medical records from 5 European countries and the USA. A multivariable logistic regression model assessed baseline and short-term (0-3 months post BI initiation) factors associated with long-term (3-24 months) glycaemic control and hypoglycaemia.
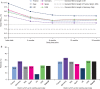
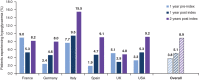
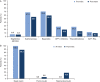
Results: Overall, 40 627 patients were included; 20.9% and 27.8% achieved the general HbA1c target of ≤7% at 3 and 24 months post BI initiation, respectively. Failure to achieve HbA1c ≤7% at 3 months was associated with increased risk of failing to achieve target at 24 months (odds ratio [OR], 3.70 [95% CI, 3.41-4.00]). Over 24 months, 8.9% of patients experienced a recorded hypoglycaemic event. Hypoglycaemia during the initial 3-month period was associated with longer-term risk of these events over the ensuing 3 to 24 months (OR, 5.71 [95% CI, 4.67-6.99]).
Conclusions: Initiating BI with or without OADs is associated with short- and long-term suboptimal glycaemic control; the majority of patients fail to achieve HbA1c target ≤7% in the first 3 months, or after 2 years of BI treatment. Treatment response and hypoglycaemia incidence by 3 months post BI initiation are associated with longer-term glycaemic control and hypoglycaemic risk, respectively. These results support the need for early anti-hyperglycaemic interventions that more effectively control blood glucose levels without increasing the risk of hypoglycaemia.
Keywords: basal insulin; glycaemic control; hypoglycaemia; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

References
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. - PubMed
-
- National Institute for Health and Clinical Excellence . NICE guideline: type 2 diabetes in adults: management. 2015. http://www.nice.org.uk/guidance/ng28. Accessed February 14, 2017.
-
- Working Group of the CPG on type 2 Diabetes 2 . Clinical practice guideline for type 2 diabetes [Guía de Práctica Clínica sobre Diabetes tipo 2]. 2010. http://www.guiasalud.es/egpc/traduccion/ingles/diabetes/completa/index.html. Accessed December 11, 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

