Binding of ZO-1 to α5β1 integrins regulates the mechanical properties of α5β1-fibronectin links
- PMID: 28251923
- PMCID: PMC5541835
- DOI: 10.1091/mbc.E17-01-0006
Binding of ZO-1 to α5β1 integrins regulates the mechanical properties of α5β1-fibronectin links
Abstract
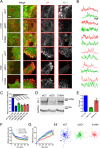
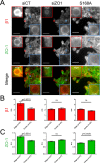
Fundamental processes in cell adhesion, motility, and rigidity adaptation are regulated by integrin-mediated adhesion to the extracellular matrix (ECM). The link between the ECM component fibronectin (fn) and integrin α5β1 forms a complex with ZO-1 in cells at the edge of migrating monolayers, regulating cell migration. However, how this complex affects the α5β1-fn link is unknown. Here we show that the α5β1/ZO-1 complex decreases the resistance to force of α5β1-fn adhesions located at the edge of migrating cell monolayers while also increasing α5β1 recruitment. Consistently with a molecular clutch model of adhesion, this effect of ZO-1 leads to a decrease in the density and intensity of adhesions in cells at the edge of migrating monolayers. Taken together, our results unveil a new mode of integrin regulation through modification of the mechanical properties of integrin-ECM links, which may be harnessed by cells to control adhesion and migration.
© 2017 González-Tarragó et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

