Salvianolic acid B inhibits mitochondrial dysfunction by up-regulating mortalin
- PMID: 28251987
- PMCID: PMC5333085
- DOI: 10.1038/srep43097
Salvianolic acid B inhibits mitochondrial dysfunction by up-regulating mortalin
Abstract
Salvianolic acid B is an antioxidative ingredient derived from Radix Salviae miltiorrhizae that has been widely used to treat liver diseases. However, the therapeutic mechanism underlying Salvianolic acid B has remained largely unknown. Our studies verified that Salvianolic acid B efficiently blocked mitochondrial deformation and dysfunction induced by H2O2 in the human hepatocyte cell line HL7702. Mortalin, a mitochondrial molecular chaperone, maintains mitochondrial morphology stabilization and function integrity. Previous results showed that mortalin overexpression has been observed in hematoma carcinoma cells and that mortalin maintains mitochondrial homeostasis and antagonizes oxidative stress damage. We found that Salvianolic acid B significantly up-regulated mortalin protein expression levels. In addition, Salvianolic acid B lost the function of preventing mitochondrial deformation and dysfunction induced by oxidative stress under mortalin knockdown conditions. We further found that mortalin overexpression increases the mRNA expression of mitofusin-related factor Mfn1 and mitofission-related factor hFis1. In conclusion, Salvianolic acid B maintains the mitochondrial structure stabilization and functional integrity by up-regulating mortalin, which may be associated with increased mitofusin factor Mfn1 and reduced mitofission factor hFis1.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
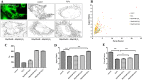

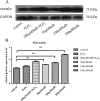
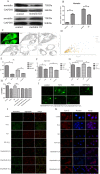
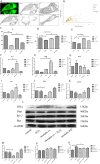
References
-
- Sanchez-Valle V., Chavez-Tapia N. C., Uribe M. & Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 19, 4850–4860 (2012). - PubMed
-
- Tian L. L. et al.. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int J Biochem Cell Biol. 40, 409–422 (2008). - PubMed
-
- Friedman S. L. Liver fibrosis - from bench to bedside. J Hepatol. 38, S38–S53 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

