Evolution of complexity in the zebrafish synapse proteome
- PMID: 28252024
- PMCID: PMC5337974
- DOI: 10.1038/ncomms14613
Evolution of complexity in the zebrafish synapse proteome
Abstract
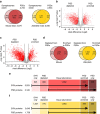
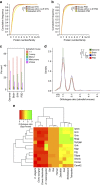
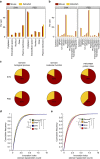
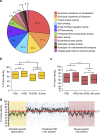
The proteome of human brain synapses is highly complex and is mutated in over 130 diseases. This complexity arose from two whole-genome duplications early in the vertebrate lineage. Zebrafish are used in modelling human diseases; however, its synapse proteome is uncharacterized, and whether the teleost-specific genome duplication (TSGD) influenced complexity is unknown. We report the characterization of the proteomes and ultrastructure of central synapses in zebrafish and analyse the importance of the TSGD. While the TSGD increases overall synapse proteome complexity, the postsynaptic density (PSD) proteome of zebrafish has lower complexity than mammals. A highly conserved set of ∼1,000 proteins is shared across vertebrates. PSD ultrastructural features are also conserved. Lineage-specific proteome differences indicate that vertebrate species evolved distinct synapse types and functions. The data sets are a resource for a wide range of studies and have important implications for the use of zebrafish in modelling human synaptic diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Bayés A. & Grant S. G. N. Neuroproteomics: understanding the molecular organization and complexity of the brain. Nat. Rev. Neurosci. 10, 635–646 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

