A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation
- PMID: 28252037
- PMCID: PMC5333160
- DOI: 10.1038/srep43522
A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation
Abstract
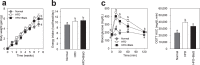
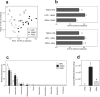
The gut microbiota is an important contributor to the worldwide prevalence of metabolic syndrome (MS), which includes obesity and diabetes. The anti-MS effects exerted by Bifidobacterium animalis ssp. lactis GCL2505 (BlaG), a highly proliferative Bifidobacterium strain in the gut, and B. longum ssp. longum JCM1217T (BloJ) were comparatively examined. BlaG treatment reduced visceral fat accumulation and improved glucose tolerance, whereas BloJ had no effect on these parameters. Gut microbial analysis revealed that BlaG exerted stronger effects on the overall bacterial structure of the gut microbiota than BloJ, including enrichment of the genus Bifidobacterium. The levels of acetate and glucagon-like peptide-1 were increased by BlaG treatment in both the gut and plasma, but not by BloJ treatment. Correlation analysis suggested that the elevation of gut acetate levels by BlaG treatment plays a pivotal role in the BlaG-induced anti-MS effects. These findings indicated that BlaG, a highly viable and proliferative probiotic, improves metabolic disorders by modulating gut microbiota, which results in the elevation of SCFAs, especially acetate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Ley R. E., Turnbaugh P. J., Klein S. & Gordon J. I. Microbial ecology: Human gut microbes associated with obesity. Nature 444, 1022–1023 (2006). - PubMed
-
- Cani P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007). - PubMed
-
- Cani P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

