A pharmacological approach in newly established retinal vein occlusion model
- PMID: 28252108
- PMCID: PMC5333144
- DOI: 10.1038/srep43509
A pharmacological approach in newly established retinal vein occlusion model
Abstract
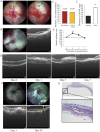
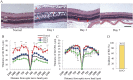
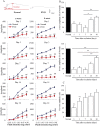
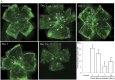
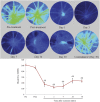
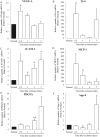
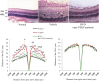
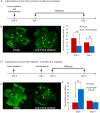
The mechanism underlying the effects of anti-vascular endothelial growth factor (VEGF) antibody in retinal vein occlusion (RVO) treatment is poorly understood, partly due to the lack of RVO animal models that mimic clinical pathology. The aims of this study were to establish a suitable RVO model, clarify the pathogenic mechanisms, and evaluate the effects of anti-VEGF antibody in the model. Mouse retinal veins were occluded by laser photocoagulation after rose bengal injection. Reduction of the b/a wave amplitude ratio, retinal nonperfusion, cystoid edema, and hard exudates were observed after occlusion, and expression of RVO-related genes was altered. Administration of anti-VEGF antibody immediately, or 7 days, after occlusion resulted in reduction and increase of the nonperfused area, respectively. We conclude that the present model will be useful for clarification of the pathogenic mechanisms, and that the timing of anti-VEGF antibody administration is important for the successful amelioration of retinal nonperfusion.
Conflict of interest statement
This investigation was supported by funding from Novartis K.K. (Tokyo, Japan).
Figures
Similar articles
-
Effect of intravitreal anti-vascular endothelial growth factor treatment on the retinal gene expression in acute experimental central retinal vein occlusion.Ophthalmic Res. 2012;47(3):157-62. doi: 10.1159/000330279. Epub 2011 Nov 24. Ophthalmic Res. 2012. PMID: 22116547
-
Creation of Retinal Vein Occlusion Model in Cynomolgus Monkeys and Determination of its Pathological Features.Curr Neurovasc Res. 2021;18(1):123-133. doi: 10.2174/1567202617999200831151118. Curr Neurovasc Res. 2021. PMID: 32867658
-
Photocoagulation of the Retinal Nonperfusion Area Prevents the Expression of the Vascular Endothelial Growth Factor in an Animal Model.Invest Ophthalmol Vis Sci. 2017 Nov 1;58(13):5946-5953. doi: 10.1167/iovs.17-22739. Invest Ophthalmol Vis Sci. 2017. PMID: 29098298
-
Anti-vascular endothelial growth factor treatment for retinal vein occlusions.Ophthalmologica. 2012;227 Suppl 1:30-5. doi: 10.1159/000337157. Epub 2012 Apr 24. Ophthalmologica. 2012. PMID: 22517123 Review.
-
A Review: Proteomics in Retinal Artery Occlusion, Retinal Vein Occlusion, Diabetic Retinopathy and Acquired Macular Disorders.Int J Mol Sci. 2017 Apr 28;18(5):907. doi: 10.3390/ijms18050907. Int J Mol Sci. 2017. PMID: 28452939 Free PMC article. Review.
Cited by
-
Complement C3/C3aR Signaling Pathway Inhibition Ameliorates Retinal Damage in Experimental Retinal Vein Occlusion.Invest Ophthalmol Vis Sci. 2025 May 1;66(5):2. doi: 10.1167/iovs.66.5.2. Invest Ophthalmol Vis Sci. 2025. PMID: 40310626 Free PMC article.
-
Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion.Molecules. 2025 Jun 17;30(12):2622. doi: 10.3390/molecules30122622. Molecules. 2025. PMID: 40572585 Free PMC article.
-
Gene expression profiling in a mouse model of retinal vein occlusion induced by laser treatment reveals a predominant inflammatory and tissue damage response.PLoS One. 2018 Mar 12;13(3):e0191338. doi: 10.1371/journal.pone.0191338. eCollection 2018. PLoS One. 2018. PMID: 29529099 Free PMC article.
-
Sphingosine-1-phosphate receptor 1/5 selective agonist alleviates ocular vascular pathologies.Sci Rep. 2024 Apr 27;14(1):9700. doi: 10.1038/s41598-024-60540-6. Sci Rep. 2024. PMID: 38678148 Free PMC article.
-
Ocular Surface Temperature Profile of Eyes with Retinal Vein Occlusion.J Clin Med. 2023 Dec 3;12(23):7479. doi: 10.3390/jcm12237479. J Clin Med. 2023. PMID: 38068531 Free PMC article.
References
-
- Bunce C., Xing W. & Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007–March 2008. Eye 24, 1692–1699 (2010). - PubMed
-
- Jaissle G. B. et al.. Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 249, 183–192 (2011). - PMC - PubMed
-
- Campochiaro P. A. et al.. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117, 1102–1112 e1101 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

