Defining the noninferiority margin and analysing noninferiority: An overview
- PMID: 28252213
- PMCID: PMC5510081
- DOI: 10.1111/bcp.13280
Defining the noninferiority margin and analysing noninferiority: An overview
Abstract
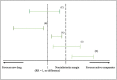
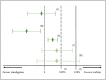
Noninferiority trials are used to assess whether the effect of a new drug is not worse than an active comparator by more than a noninferiority margin. If the difference between the new drug and the active comparator does not exceed this prespecified margin, noninferiority can be concluded. This margin must be specified based on clinical and statistical reasoning; however, it is considered as one of the most challenging steps in the design of noninferiority trials. Regulators recommend that the margin should be defined based on the historical evidence of the active comparator (the latter is often the well-established standard treatment of the disease), which can be performed by different approaches. There are several factors and assumptions that need to be accounted for during the process of defining the margin and during the analysis of noninferiority. Three methods are commonly used to analyse noninferiority trials: the fixed-margin method; the point-estimate method; and the synthesis method. This article provides an overview of analysing noninferiority and choosing the noninferiority margin.
Keywords: biostatistics; clinical trials; drug regulation; methodology; randomized controlled trials.
© 2017 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures

References
-
- ICH Expert Working Group . ICH harmonised tripartite guideline: choice of control group in clinical trials (E 10) [online]. 2000. Available from: URL: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff... (last accessed 01 February 2016).
-
- Center for Biologics Evaluation and Research (CBER) , Center for Drug Evaluation and Research (CDER) , Food and Drug Administration, U.S. Department of Health and Human Services . Guidance for industry non‐inferiority clinical trials [online]. 2010; Available from: URL:http://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf (last accessed 01 February 2016).
-
- Rothmann MD, Wiens BL, Chan IS. Design and analysis of non‐inferiority trials. Boca Raton, Florida: Chapman & Hall/CRC, 2012.
-
- Kaul S, Diamond GA. Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 2006; 145: 62–69 doi: 145/1/62 [pii]. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

