Effects of Transendocardial Stem Cell Injection on Ventricular Proarrhythmia in Patients with Ischemic Cardiomyopathy: Results from the POSEIDON and TAC-HFT Trials
- PMID: 28252842
- PMCID: PMC5442721
- DOI: 10.1002/sctm.16-0328
Effects of Transendocardial Stem Cell Injection on Ventricular Proarrhythmia in Patients with Ischemic Cardiomyopathy: Results from the POSEIDON and TAC-HFT Trials
Abstract
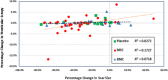
Transendocardial stem cell injection in patients with ischemic cardiomyopathy (ICM) improves left ventricular function and structure but has ill-defined effects on ventricular arrhythmias. We hypothesized that mesenchymal stem cell (MSC) implantation is not proarrhythmic. Post hoc analyses were performed on ambulatory ECGs collected from the POSEIDON and TAC-HFT trials. Eighty-eight subjects (mean age 61 ± 10 years) with ICM (mean EF 32.2% ± 9.8%) received treatment with MSC (n = 48), Placebo (n = 21), or bone marrow mononuclear cells (BMC) (n = 19). Heart rate variability (HRV) and ventricular ectopy (VE) were evaluated over 12 months. VE did not change in any group following MSC implantation. However, in patients with ≥ 1 VE run (defined as ≥ 3 consecutive premature ventricular complexes in 24 hours) at baseline, there was a decrease in VE runs at 12 months in the MSC group (p = .01), but not in the placebo group (p = .07; intergroup comparison: p = .18). In a subset of the MSC group, HRV measures of standard deviation of normal intervals was 75 ± 30 msec at baseline and increased to 87 ± 32 msec (p =.02) at 12 months, and root mean square of intervals between successive complexes was 36 ± 30 msec and increased to 58.2 ± 50 msec (p = .01) at 12 months. In patients receiving MSCs, there was no evidence for ventricular proarrhythmia, manifested by sustained or nonsustained ventricular ectopy or worsened HRV. Signals of improvement in ventricular arrhythmias and HRV in the MSC group suggest a need for further studies of the antiarrhythmic potential of MSCs. Stem Cells Translational Medicine 2017;6:1366-1372.
Keywords: Cardiac arrhythmias; Cardiomyopathy; Heart failure; Stem cells; Ventricular tachycardia.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



References
-
- McMurray JJ, Adamopoulos S, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763–776. - PubMed
-
- Strauer BE, Yousef M, Schannwell CM. The acute and long‐term effects of intracoronary stem cell transplantation in 191 patients with chronic heARt failure: The STAR‐heart study. Eur J Heart Fail 2010;12:721–729. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

