Cancer Screening Test Use - United States, 2015
- PMID: 28253225
- PMCID: PMC5657895
- DOI: 10.15585/mmwr.mm6608a1
Cancer Screening Test Use - United States, 2015
Abstract
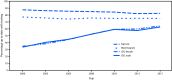
Healthy People 2020 (HP2020) includes objectives to increase screening for breast, cervical, and colorectal cancer (1) as recommended by the U.S. Preventive Services Task Force (USPSTF).* Progress toward meeting these objectives is monitored by measuring cancer screening test use against national targets using data from the National Health Interview Survey (NHIS) (1). Analysis of 2015 NHIS data indicated that screening test use remains substantially below HP2020 targets for selected cancer screening tests. Although colorectal cancer screening test use increased from 2000 to 2015, no improvements in test use were observed for breast and cervical cancer screening. Disparities exist in screening test use by race/ethnicity, socioeconomic status, and health care access indicators. Increased measures to implement evidence-based interventions and conduct targeted outreach are needed if the HP2020 targets for cancer screening are to be achieved and the disparities in screening test use are to be reduced.
Figures
References
-
- Office of Disease Prevention and Health Promotion. Healthy people 2020. Washington, DC: US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2017. https://www.healthypeople.gov
-
- National Center for Health Statistics. National Health Interview Survey, 2015. Hyattsville, Maryland: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

