Nod2: The intestinal gate keeper
- PMID: 28253332
- PMCID: PMC5333895
- DOI: 10.1371/journal.ppat.1006177
Nod2: The intestinal gate keeper
Abstract
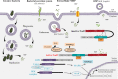
Nucleotide-binding oligomerization domain 2 (NOD2) is an intracellular pattern recognition receptor that senses bacterial peptidoglycan (PGN)-conserved motifs in cytosol and stimulates host immune response. The association of NOD2 mutations with a number of inflammatory pathologies, including Crohn disease (CD), Graft-versus-host disease (GVHD), and Blau syndrome, highlights its pivotal role in host-pathogen interactions and inflammatory response. Stimulation of NOD2 by its ligand (muramyl dipeptide) activates pro-inflammatory pathways such as nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), and Caspase-1. A loss of NOD2 function may result in a failure in the control of microbial infection, thereby initiating systemic responses and aberrant inflammation. Because the ligand of Nod2 is conserved in both gram-positive and gram-negative bacteria, NOD2 detects a wide variety of microorganisms. Furthermore, current literature evidences that NOD2 is also able to control viruses' and parasites' infections. In this review, we present and discuss recent developments about the role of NOD2 in shaping the gut commensal microbiota and pathogens, including bacteria, viruses, and parasites, and the mechanisms by which Nod2 mutations participate in disease occurrence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

