Adjuvant Capecitabine in Combination With Docetaxel, Epirubicin, and Cyclophosphamide for Early Breast Cancer: The Randomized Clinical FinXX Trial
- PMID: 28253390
- PMCID: PMC5824321
- DOI: 10.1001/jamaoncol.2016.6120
Adjuvant Capecitabine in Combination With Docetaxel, Epirubicin, and Cyclophosphamide for Early Breast Cancer: The Randomized Clinical FinXX Trial
Abstract
Importance: Capecitabine is not considered a standard agent in the adjuvant treatment of early breast cancer. The results of this study suggest that addition of adjuvant capecitabine to a regimen that contains docetaxel, epirubicin, and cyclophosphamide improves survival outcomes of patients with triple-negative breast cancer (TNBC).
Objective: To investigate the effect of capecitabine on long-term survival outcomes of patients with early breast cancer, particularly in subgroups defined by cancer estrogen receptor (ER) and progesterone receptor (PR) content, and HER2 content (human epidermal growth factor receptor 2).
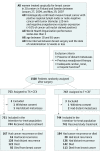
Design, setting, and participants: This is an exploratory analysis of the multicenter FinXX randomized clinical trial that accrued 1500 women in Finland and Sweden between January 27, 2004, and May 29, 2007. About half received 3 cycles of docetaxel followed by 3 cycles of cyclophosphamide, epirubicin, and fluorouracil (T+CEF), while the other half received 3 cycles of docetaxel plus capecitabine followed by 3 cycles of cyclophosphamide, epirubicin, and capecitabine (TX+CEX). Data analysis took place between January 27, 2004, and December 31, 2015.
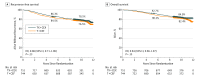
Main outcomes and measures: Recurrence-free survival (RFS).
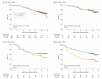
Results: Following random allocation, 747 women received T+CEF, and 753 women received TX+CEX. Five patients were excluded from the intention-to-treat population (3 had overt distant metastases at the time of randomization; 2 withdrew consent). The median age of the remaining 1495 patients was 53 years at the time of study entry; 157 (11%) had axillary node-negative disease; 1142 (76%) had ER-positive cancer; and 282 (19%) had HER2-positive cancer. The median follow-up time after random allocation was 10.3 years. There was no significant difference in RFS or overall survival between the groups (hazard ratio [HR], 0.88; 95% CI, 0.71-1.08; P = .23; and HR, 0.84, 95% CI, 0.66-1.07; P = .15; respectively). Breast cancer-specific survival tended to favor the capecitabine group (HR, 0.79; 95% CI, 0.60-1.04; P = .10). When RFS and survival of the patients were compared within the subgroups defined by cancer steroid hormone receptor status (ER and/or PR positive vs ER and PR negative) and HER2 status (positive vs negative), TX+CEX was more effective than T+CEF in the subset of patients with TNBC (HR, 0.53; 95% CI, 0.31-0.92; P = .02; and HR, 0.55, 95% CI, 0.31-0.96; P = .03; respectively).
Conclusions and relevance: Capecitabine administration with docetaxel, epirubicin, and cyclophosphamide did not prolong RFS or survival compared with a regimen that contained only standard agents. Patients with TNBC had favorable survival outcomes when treated with the capecitabine-containing regimen in an exploratory subgroup analysis.
Trial registration: clinicaltrials.gov Identifier: NCT00114816.
Conflict of interest statement
Figures

Comment in
-
Taxane Followed by Anthracycline or Vice Versa: Impact of Sequential Order on Breast Cancer Recurrence?-Reply.JAMA Oncol. 2018 Mar 1;4(3):423-424. doi: 10.1001/jamaoncol.2017.3352. JAMA Oncol. 2018. PMID: 29075758 No abstract available.
-
Taxane Followed by Anthracycline or Vice Versa: Impact of Sequential Order on Breast Cancer Recurrence?JAMA Oncol. 2018 Mar 1;4(3):423. doi: 10.1001/jamaoncol.2017.3349. JAMA Oncol. 2018. PMID: 29075767 No abstract available.
References
-
- Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274-1281. - PubMed
-
- Endo M, Shinbori N, Fukase Y, et al. Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5′-deoxy-5-fluorouridine by cyclophosphamide in mammary tumor models. Int J Cancer. 1999;83(1):127-134. - PubMed
-
- Fujimoto-Ouchi K, Tanaka Y, Tominaga T. Schedule dependency of antitumor activity in combination therapy with capecitabine/5′-deoxy-5-fluorouridine and docetaxel in breast cancer models. Clin Cancer Res. 2001;7(4):1079-1086. - PubMed
-
- O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20(12):2812-2823. - PubMed
-
- Campone M, Dobrovolskaya N, Tjulandin S, et al. A three-arm randomized phase II study of oral vinorelbine plus capecitabine versus oral vinorelbine and capecitabine in sequence versus docetaxel plus capecitabine in patients with metastatic breast cancer previously treated with anthracyclines. Breast J. 2013;19(3):240-249. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

