Effectiveness of robot-assisted gait training in children with cerebral palsy: a bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT)
- PMID: 28253887
- PMCID: PMC5333417
- DOI: 10.1186/s12887-017-0815-y
Effectiveness of robot-assisted gait training in children with cerebral palsy: a bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT)
Abstract
Background: Walking ability is a priority for many children with cerebral palsy (CP) and their parents when considering domains of importance regarding treatment interventions. Partial body-weight supported treadmill training has become an established therapeutic treatment approach to address this demand. Further, new robotic rehabilitation technologies have increasingly been implemented in the clinical setting to allow for longer training sessions with increased step repetitions while maintaining a consistent movement pattern. But the current evidence about its clinical effectiveness in pediatric rehabilitation is weak. The aim of this research project is therefore to investigate the effectiveness of robot-assisted gait training on improvements of functional gait parameters in children with cerebral palsy.
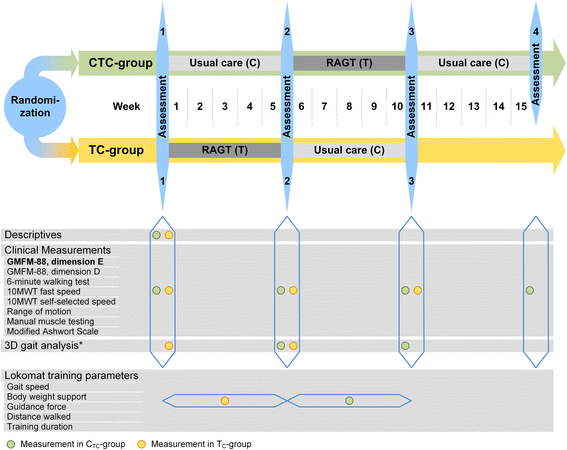
Methods/design: Children aged 6 to 18 years with bilateral spastic cerebral palsy who are able to walk at least 14 m with or without walking aids will be recruited in two pediatric therapy centers in Switzerland. Within a pragmatic cross-over design with randomized treatment sequences, they perform 5 weeks of robot-assisted gait training (three times per week with a maximum of 45 min walking time each) or a 5-week period of standard treatment, which is individually customized to the needs of the child and usually consists of 1-2 sessions of physiotherapy per week and additional hippotherapy, circuit training as well as occupational therapy as necessary. Both interventions take place in an outpatient setting. The percentage score of the dimension E of the Gross Motor Function Measure-88 (GMFM-88) as primary outcome as well as the dimension D of the GMFM-88, 6-minute and 10-meter walking tests as secondary outcomes are assessed before and at the end of each intervention period. Additionally, a 5-week follow-up assessment is scheduled for the children who are assigned to the standard treatment first. Treatment effects, period effects as well as follow-up effects are analyzed with paired analyses and independent test statistics are used to assess carry-over effects.
Discussion: Although robot-assisted gait training has become an established treatment option to address gait impairments, evidence for its effectiveness is vague. This pragmatic trial will provide important information on its effects under clinical outpatient conditions.
Trial registration: ClinicalTrials.gov: NCT00887848 . Registered 23 April 2009.
Keywords: Adolescent; Cerebral palsy; Child; Cross-over design; Randomized controlled trial; Robotics; Therapy; Walking.
Figures


References
-
- Beveridge B, Feltracco D, Struyf J, Strauss E, Dang S, Phelan S et al. “You gotta try it all”: Parents’ Experiences with Robotic Gait Training for their Children with Cerebral Palsy. Phys Occup Ther Pediatr. 2015;35(4):327-41. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

