Rapid and stable changes in maturation-related phenotypes of the adult hippocampal neurons by electroconvulsive treatment
- PMID: 28253930
- PMCID: PMC5335812
- DOI: 10.1186/s13041-017-0288-9
Rapid and stable changes in maturation-related phenotypes of the adult hippocampal neurons by electroconvulsive treatment
Abstract
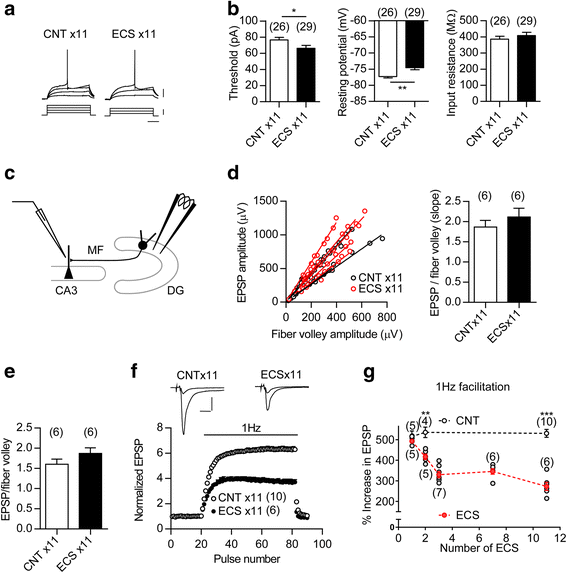
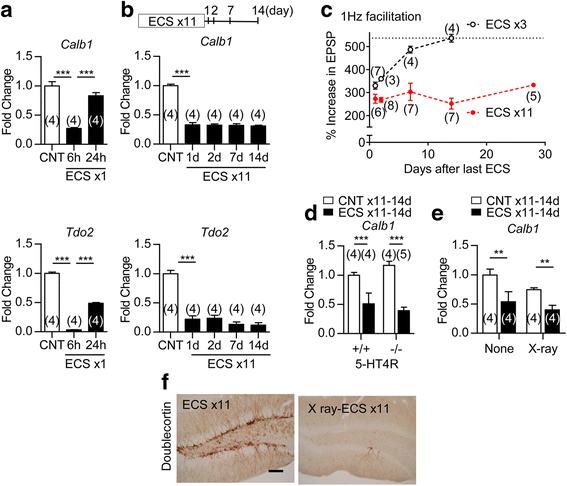
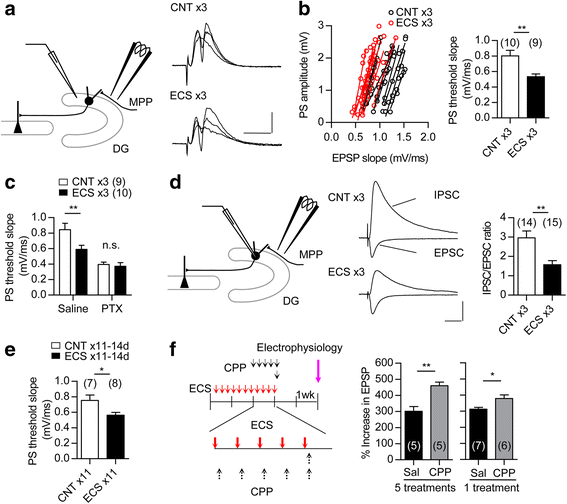
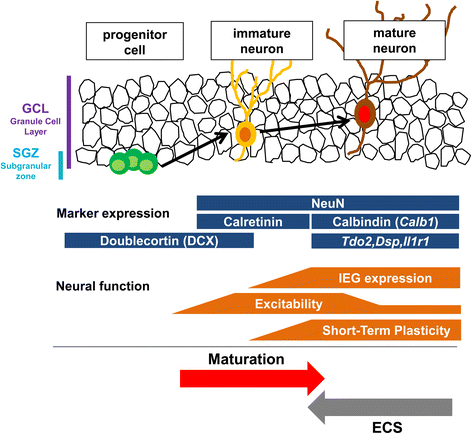
Electroconvulsive therapy (ECT) is a highly effective and fast-acting treatment for depression. Despite a long history of clinical use, its mechanism of action remains poorly understood. Recently, a novel cellular mechanism of antidepressant action has been proposed: the phenotype of mature brain neurons is transformed to immature-like one by antidepressant drug treatments. We show here that electroconvulsive stimulation (ECS), an animal model of ECT, causes profound changes in maturation-related phenotypes of neurons in the hippocampal dentate gyrus of adult mice. Single ECS immediately reduced expression of mature neuronal markers in almost entire population of dentate granule cells. After ECS treatments, granule cells showed some of physiological properties characteristic of immature granule cells such as higher somatic intrinsic excitability and smaller frequency facilitation at the detate-to-CA3 synapse. The rapid downregulation of maturation markers was suppressed by antagonizing glutamate NMDA receptors, but not by perturbing the serotonergic system. While single ECS caused short-lasting effects, repeated ECS induced stable changes in the maturation-related phenotypes lasting more than 2 weeks along with enhancement of synaptic excitation of granule cells. Augmentation of synaptic inhibition or blockade of NMDA receptors after repeated ECS facilitated regaining the initial mature phenotype, suggesting a role for endogenous neuronal excitation in maintaining the altered maturation-related phenotype probably via NMDA receptor activation. These results suggest that brief neuronal activation by ECS induces "dematuration" of the mature granule cells and that enhanced endogenous excitability is likely to support maintenance of such a demature state. The global increase in neuronal excitability accompanying this process may be relevant to the high efficacy of ECT.
Keywords: Antidepressant; Electroconvulsive seizure; Granule cell; Hippocampus; Maturation.
Figures





References
-
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2003;174:151-62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

