Identification of a novel CNTNAP1 mutation causing arthrogryposis multiplex congenita with cerebral and cerebellar atrophy
- PMID: 28254648
- PMCID: PMC5569911
- DOI: 10.1016/j.ejmg.2017.02.006
Identification of a novel CNTNAP1 mutation causing arthrogryposis multiplex congenita with cerebral and cerebellar atrophy
Abstract
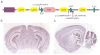
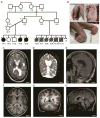
Arthrogryposis multiplex congenital, the occurrence of multiple joint contractures at birth, can in some cases be accompanied by insufficient myelination of peripheral nerves, muscular hypotonia, reduced tendon reflexes, and respiratory insufficiency. Recently mutations in the CASPR/CNTN1 complex have been associated with similar severe phenotypes and CNTNAP1 gene mutations, causing loss of the CASPR protein, were shown to cause severe, prenatal onset arthrogryposis multiplex congenita in four unrelated families. Here we report a consanguineous Arab family from Qatar with three children having an early lethal form of arthrogryposis multiplex congenita and a novel frameshift mutation in CNTNAP1. We further expand the existing CNTNAP1-associated phenotype to include profound cerebral and cerebellar atrophy.
Keywords: Arab; Arthrogryposis multiplex congenita; CNTNAP1; Cerebral atrophy; Qatar.
Copyright © 2017 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Figures
References
-
- Hall JG. Arthrogryposis (multiple congenital contractures): diagnostic approach to etiology, classification, genetics, and general principles. European journal of medical genetics. 2014;57:464–472. - PubMed
-
- Hoff JM, Loane M, Gilhus NE, et al. Arthrogryposis multiplexa congenita: an epidemiologic study of nearly 9 million births in 24 EUROCAT registers. European journal of obstetrics, gynecology, and reproductive biology. 2011;159:347–350. - PubMed
-
- Laquerriere A, Maluenda J, Camus A, et al. Mutations in CNTNAP1 and ADCY6 are responsible for severe arthrogryposis multiplex congenita with axoglial defects. Hum Mol Genet. 2014;23:2279–2289. - PubMed
-
- Bonnon C, Goutebroze L, Denisenko-Nehrbass N, et al. The paranodal complex of F3/contactin and caspr/paranodin traffics to the cell surface via a non-conventional pathway. The Journal of biological chemistry. 2003;278:48339–48347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

