Cytokinin-Mediated Regulation of Reactive Oxygen Species Homeostasis Modulates Stomatal Immunity in Arabidopsis
- PMID: 28254779
- PMCID: PMC5385949
- DOI: 10.1105/tpc.16.00583
Cytokinin-Mediated Regulation of Reactive Oxygen Species Homeostasis Modulates Stomatal Immunity in Arabidopsis
Abstract
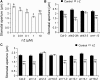
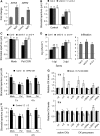
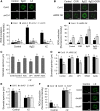
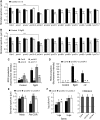
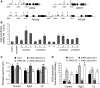
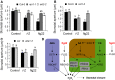
Stomata play an important role in preinvasive defense responses by limiting pathogen entry into leaves. Although the stress hormones salicylic acid (SA) and abscisic acid (ABA) are known to regulate stomatal immunity, the role of growth promoting hormones is far from understood. Here, we show that in Arabidopsis thaliana, cytokinins (CKs) function in stomatal defense responses. The cytokinin receptor HISTIDINE KINASE3 (AHK3) and RESPONSE REGULATOR2 (ARR2) promote stomatal closure triggered by pathogen-associated molecular pattern (PAMP) and resistance to Pseudomonas syringae pv tomato bacteria. Importantly, the cytokinin trans-zeatin induces stomatal closure and accumulation of reactive oxygen species (ROS) in guard cells through AHK3 and ARR2 in an SA-dependent and ABA-independent manner. Using pharmacological and reverse genetics approaches, we found that CK-mediated stomatal responses involve the apoplastic peroxidases PRX4, PRX33, PRX34, and PRX71, but not the NADPH oxidases RBOHD and RBOHF. Moreover, ARR2 directly activates the expression of PRX33 and PRX34, which are required for SA- and PAMP-triggered ROS production. Thus, the CK signaling pathway regulates ROS homeostasis in guard cells, which leads to enhanced stomatal immunity and plant resistance to bacteria.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures


References
-
- Acharya B.R., Assmann S.M. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69: 451–462. - PubMed
-
- Arnaud D., Hwang I. (2015). A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant 8: 566–581. - PubMed
-
- Bolwell G.P., Bindschedler L.V., Blee K.A., Butt V.S., Davies D.R., Gardner S.L., Gerrish C., Minibayeva F. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53: 1367–1376. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

