β-Cell Inactivation of Gpr119 Unmasks Incretin Dependence of GPR119-Mediated Glucoregulation
- PMID: 28254842
- PMCID: PMC5860191
- DOI: 10.2337/db17-0017
β-Cell Inactivation of Gpr119 Unmasks Incretin Dependence of GPR119-Mediated Glucoregulation
Abstract
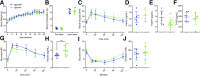
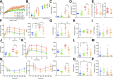
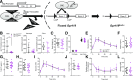
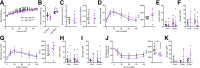
GPR119 was originally identified as an orphan β-cell receptor; however, subsequent studies demonstrated that GPR119 also regulates β-cell function indirectly through incretin hormone secretion. We assessed the importance of GPR119 for β-cell function in Gpr119-/- mice and in newly generated Gpr119βcell-/- mice. Gpr119-/- mice displayed normal body weight and glucose tolerance on a regular chow (RC) diet. After high-fat feeding, Gpr119-/- mice exhibited reduced fat mass, decreased levels of circulating adipokines, improved insulin sensitivity, and better glucose tolerance. Unexpectedly, oral and intraperitoneal glucose tolerance and the insulin response to glycemic challenge were not perturbed in Gpr119βcell-/- mice on RC and high-fat diets. Moreover, islets from Gpr119-/- and Gpr119βcell-/- mice exhibited normal insulin responses to glucose and β-cell secretagogues. Furthermore, the selective GPR119 agonist AR231453 failed to directly enhance insulin secretion from perifused islets. In contrast, AR231453 increased plasma glucagon-like peptide 1 (GLP-1) and insulin levels and improved glucose tolerance in wild-type and Gpr119βcell-/- mice. These findings demonstrate that β-cell GPR119 expression is dispensable for the physiological control of insulin secretion and the pharmacological response to GPR119 agonism, findings that may inform the lack of robust efficacy in clinical programs assessing GPR119 agonists for the therapy of type 2 diabetes.
© 2017 by the American Diabetes Association.
Figures


Similar articles
-
GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms.Endocrinology. 2011 Feb;152(2):374-83. doi: 10.1210/en.2010-1047. Epub 2010 Nov 10. Endocrinology. 2011. PMID: 21068156 Free PMC article.
-
A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release.Endocrinology. 2008 May;149(5):2038-47. doi: 10.1210/en.2007-0966. Epub 2008 Jan 17. Endocrinology. 2008. PMID: 18202141
-
A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release.Endocrinology. 2007 Jun;148(6):2601-9. doi: 10.1210/en.2006-1608. Epub 2007 Feb 8. Endocrinology. 2007. PMID: 17289847
-
The therapeutic potential of GPR119 agonists for type 2 diabetes.Expert Opin Investig Drugs. 2012 Mar;21(3):321-8. doi: 10.1517/13543784.2012.657797. Epub 2012 Feb 3. Expert Opin Investig Drugs. 2012. PMID: 22292451 Review.
-
GPR119 agonists 2009-2011.Pharm Pat Anal. 2012 Jul;1(3):285-99. doi: 10.4155/ppa.12.33. Pharm Pat Anal. 2012. PMID: 24236842 Review.
Cited by
-
Targeting the GPR119/incretin axis: a promising new therapy for metabolic-associated fatty liver disease.Cell Mol Biol Lett. 2021 Jul 7;26(1):32. doi: 10.1186/s11658-021-00276-7. Cell Mol Biol Lett. 2021. PMID: 34233623 Free PMC article. Review.
-
Bidirectional GPR119 Agonism Requires Peptide YY and Glucose for Activity in Mouse and Human Colon Mucosa.Endocrinology. 2018 Apr 1;159(4):1704-1717. doi: 10.1210/en.2017-03172. Endocrinology. 2018. PMID: 29471473 Free PMC article.
-
Potential metabolic and behavioural roles of the putative endocannabinoid receptors GPR18, GPR55 and GPR119 in feeding.Curr Neuropharmacol. 2019;17(10):947-960. doi: 10.2174/1570159X17666190118143014. Curr Neuropharmacol. 2019. PMID: 31146657 Free PMC article. Review.
-
Targeting lipid GPCRs to treat type 2 diabetes mellitus - progress and challenges.Nat Rev Endocrinol. 2021 Mar;17(3):162-175. doi: 10.1038/s41574-020-00459-w. Epub 2021 Jan 25. Nat Rev Endocrinol. 2021. PMID: 33495605 Review.
-
Connecting pancreatic islet lipid metabolism with insulin secretion and the development of type 2 diabetes.Ann N Y Acad Sci. 2020 Feb;1461(1):53-72. doi: 10.1111/nyas.14037. Epub 2019 Apr 2. Ann N Y Acad Sci. 2020. PMID: 30937918 Free PMC article. Review.
References
-
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014;2:56–64 - PubMed
-
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–837 - PubMed
-
- Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

