Ketamine as the anaesthetic for electroconvulsive therapy: the KANECT randomised controlled trial
- PMID: 28254962
- PMCID: PMC5451642
- DOI: 10.1192/bjp.bp.116.189134
Ketamine as the anaesthetic for electroconvulsive therapy: the KANECT randomised controlled trial
Erratum in
-
Ketamine as the anaesthetic for electroconvulsive therapy: the KANECT randomised controlled trial - CORRIGENDUM.Br J Psychiatry. 2018 May;212(5):323. doi: 10.1192/bjp.2018.76. Epub 2018 Apr 6. Br J Psychiatry. 2018. PMID: 29622052 Free PMC article.
Abstract
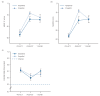
BackgroundKetamine has recently become an agent of interest as an acute treatment for severe depression and as the anaesthetic for electroconvulsive therapy (ECT). Subanaesthetic doses result in an acute reduction in depression severity while evidence is equivocal for this antidepressant effect with anaesthetic or adjuvant doses. Recent systematic reviews call for high-quality evidence from further randomised controlled trials (RCTs).AimsTo establish if ketamine as the anaesthetic for ECT results in fewer ECT treatments, improvements in depression severity ratings and less memory impairment than the standard anaesthetic.MethodDouble-blind, parallel-design, RCT of intravenous ketamine (up to 2 mg/kg) with an active comparator, intravenous propofol (up to 2.5 mg/kg), as the anaesthetic for ECT in patients receiving ECT for major depression on an informal basis. (Trial registration: European Clinical Trials Database (EudraCT): 2011-000396-14 and clinicalTrials.gov: NCT01306760)ResultsNo significant differences were found on any outcome measure during, at the end of or 1 month following the ECT course.ConclusionsKetamine as an anaesthetic does not enhance the efficacy of ECT.
© The Royal College of Psychiatrists 2017.
Conflict of interest statement
Declaration of interestC.AS. reports grants from Vifor Pharma, outside the submitted work. I.C.R. (deceased) declared personal fees from AstraZeneca, Sanofi Aventis and Sunovion, and non-financial support from Lundbeck, between 2009 and 2014 and all outside the submitted work.
Figures
References
-
- World Health Organization Depression Fact sheet N369. WHO, 2015. (http://www.who.int/mediacentre/factsheets/fs369/en/).
-
- Waite J, Easton A. The ECT Handbook (3rd edn). Royal College of Psychiatrists, 2013.
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–64. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

