Histone deacetylase 6 regulates cytokinesis and erythrocyte enucleation through deacetylation of formin protein mDia2
- PMID: 28255013
- PMCID: PMC5451330
- DOI: 10.3324/haematol.2016.161513
Histone deacetylase 6 regulates cytokinesis and erythrocyte enucleation through deacetylation of formin protein mDia2
Abstract
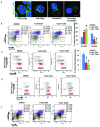
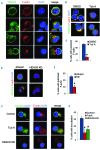
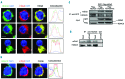
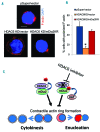
The formin protein mDia2 plays a critical role in a number of cellular processes through its ability to promote nucleation and elongation of actin filaments. In erythroblasts, this includes control of cytokinesis and enucleation by regulating contractile actin ring formation. Here we report a novel mechanism of how mDia2 is regulated: through acetylation and deacetylation at lysine 970 in the formin homology 2 domain. Ectopic expression of an acetyl-mimic mDia2 mutant in mouse erythroblasts is sufficient to abolish contractile actin ring formation at the cleavage furrow and subsequent erythrocyte cytokinesis and enucleation. We also identified that class II histone deacetylase 6 deacetylates and subsequently activates mDia2. Knockdown or inhibition of histone deacetylase 6 impairs contractile actin ring formation, and expression of a non-acetyl-mimic mDia2 mutant restores the contractile actin ring and rescues the impairment of enucleation. In addition to revealing a new step in mDia2 regulation, this study may unveil a novel regulatory mechanism of formin-mediated actin assembly, since the K970 acetylation site is conserved among Dia proteins.
Copyright© Ferrata Storti Foundation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

