Myocardial aging as a T-cell-mediated phenomenon
- PMID: 28255084
- PMCID: PMC5373357
- DOI: 10.1073/pnas.1621047114
Myocardial aging as a T-cell-mediated phenomenon
Abstract
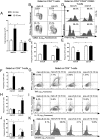
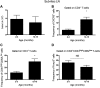
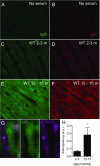
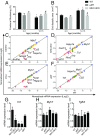
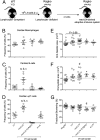
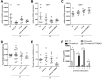
In recent years, the myocardium has been rediscovered under the lenses of immunology, and lymphocytes have been implicated in the pathogenesis of cardiomyopathies with different etiologies. Aging is an important risk factor for heart diseases, and it also has impact on the immune system. Thus, we sought to determine whether immunological activity would influence myocardial structure and function in elderly mice. Morphological, functional, and molecular analyses revealed that the age-related myocardial impairment occurs in parallel with shifts in the composition of tissue-resident leukocytes and with an accumulation of activated CD4+ Foxp3- (forkhead box P3) IFN-γ+ T cells in the heart-draining lymph nodes. A comprehensive characterization of different aged immune-deficient mouse strains revealed that T cells significantly contribute to age-related myocardial inflammation and functional decline. Upon adoptive cell transfer, the T cells isolated from the mediastinal lymph node (med-LN) of aged animals exhibited increased cardiotropism, compared with cells purified from young donors or from other irrelevant sites. Nevertheless, these cells caused rather mild effects on cardiac functionality, indicating that myocardial aging might stem from a combination of intrinsic and extrinsic (immunological) factors. Taken together, the data herein presented indicate that heart-directed immune responses may spontaneously arise in the elderly, even in the absence of a clear tissue damage or concomitant infection. These observations might shed new light on the emerging role of T cells in myocardial diseases, which primarily affect the elderly population.
Keywords: T cells; aging; inflammaging; inflammation; myocardial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

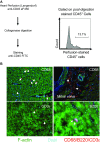

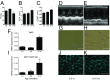
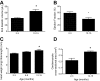
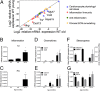



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

