Hepatitis C treatment: where are we now?
- PMID: 28255252
- PMCID: PMC5322849
- DOI: 10.2147/IJGM.S127689
Hepatitis C treatment: where are we now?
Abstract
Chronic hepatitis C infection affects millions of people worldwide and confers significant morbidity and mortality. Effective treatment is needed to prevent disease progression and associated complications. Previous treatment options were limited to interferon and ribavirin (RBV) regimens, which gave low cure rates and were associated with unpleasant side effects. The era of direct-acting antiviral (DAA) therapies began with the development of first-generation NS3/4A protease inhibitors in 2011. They vastly improved outcomes for patients, particularly those with genotype 1 infection, the most prevalent genotype globally. Since then, a multitude of DAAs have been licensed for use, and outcomes for patients have improved further, with fewer side effects and cure rates approaching 100%. Recent regimens are interferon-free, and in many cases, RBV-free, and involve a combination of DAA agents. This review summarizes the treatment options currently available and discusses potential barriers that may delay the global eradication of hepatitis C.
Keywords: directly acting antivirals; hepatitis C; hepatitis C eradication; interferon-free regimens; protease inhibitors; ribavirin-free regimens.
Conflict of interest statement
Disclosure SDT-R holds grants from the UK Medical Research Council and the Wellcome Trust (London, UK). The authors report no conflicts of interest in this work.
Figures
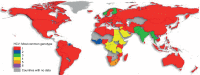
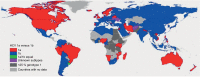

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

