Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis
- PMID: 28255275
- PMCID: PMC5332877
- DOI: 10.7150/ijbs.16951
Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis
Abstract
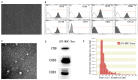
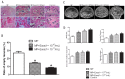
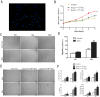
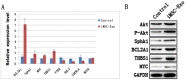
Background: Local ischemia is the main pathological performance in osteonecrosis of the femoral head (ONFH). There is currently no effective therapy to promote angiogenesis in the femoral head. Recent studies revealed that exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells (iPS-MSC-Exos) have great therapeutic potential in ischemic tissues, but whether they could promote angiogenesis in ONFH has not been reported, and little is known regarding the underlying mechanism. Methods: iPS-MSC-Exos were intravenously injected to a steroid-induced rat osteonecrosis model. Samples of the femoral head were obtained 3 weeks after all the injections. The effects were assessed by measuring local angiogenesis and bone loss through histological and immunohistochemical (IHC) staining, micro-CT and three-dimensional microangiography. The effects of exosomes on endothelial cells were studied through evaluations of proliferation, migration and tube-forming analyses. The expression levels of angiogenic related PI3K/Akt signaling pathway of endothelial cells were evaluated following stimulation of iPS-MSC-Exos. The promoting effects of exosomes were re-evaluated following blockade of PI3K/Akt. Results: The in vivo study revealed that administration of iPS-MSC-Exos significantly prevented bone loss, and increased microvessel density in the femoral head compared with control group. We found that iPS-MSC-Exos significantly enhanced the proliferation, migration and tube-forming capacities of endothelial cells in vitro. iPS-MSC-Exos could activate PI3K/Akt signaling pathway in endothelial cells. Moreover, the promoting effects of iPS-MSC-Exos were abolished after blockade of PI3K/Akt on endothelial cells. Conclusions: Our findings suggest that transplantation of iPS-MSC-Exos exerts a preventative effect on ONFH by promoting local angiogenesis and preventing bone loss. The promoting effect might be attributed to activation of the PI3K/Akt signaling pathway on endothelial cells. The data provide the first evidence for the potential of iPS-MSC-Exos in treating ONFH.
Keywords: Angiogenesis.; Exosomes; Femoral head; Osteonecrosis; induced pluripotent stem cells (iPSCs).
Conflict of interest statement
Conflicts of Interest: Xiaolin Liu, Qing Li, Xin Niu, Bin Hu, Shengbao Chen, Wenqi Song, Jian Ding, Changqing Zhang and Yang Wang declare that they have no conflict of interest.
Figures

Similar articles
-
Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis.J Transl Med. 2015 Feb 1;13:49. doi: 10.1186/s12967-015-0417-0. J Transl Med. 2015. PMID: 25638205 Free PMC article.
-
Exosomes derived from human CD34+ stem cells transfected with miR-26a prevent glucocorticoid-induced osteonecrosis of the femoral head by promoting angiogenesis and osteogenesis.Stem Cell Res Ther. 2019 Nov 15;10(1):321. doi: 10.1186/s13287-019-1426-3. Stem Cell Res Ther. 2019. PMID: 31730486 Free PMC article.
-
Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats.Int J Biol Sci. 2016 May 25;12(7):836-49. doi: 10.7150/ijbs.14809. eCollection 2016. Int J Biol Sci. 2016. PMID: 27313497 Free PMC article.
-
Therapeutic Potential of Exosomes Derived from Adipose Tissue-Sourced Mesenchymal Stem Cells in the Treatment of Neural and Retinal Diseases.Int J Mol Sci. 2022 Apr 19;23(9):4487. doi: 10.3390/ijms23094487. Int J Mol Sci. 2022. PMID: 35562878 Free PMC article. Review.
-
Research Progress on Exosomes in Osteonecrosis of the Femoral Head.Orthop Surg. 2022 Sep;14(9):1951-1957. doi: 10.1111/os.13393. Epub 2022 Aug 4. Orthop Surg. 2022. PMID: 35924692 Free PMC article. Review.
Cited by
-
The application potential of iMSCs and iMSC-EVs in diseases.Front Bioeng Biotechnol. 2024 Jul 29;12:1434465. doi: 10.3389/fbioe.2024.1434465. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39135947 Free PMC article. Review.
-
Cortistatin prevents glucocorticoid-associated osteonecrosis of the femoral head via the GHSR1a/Akt pathway.Commun Biol. 2024 Jan 26;7(1):132. doi: 10.1038/s42003-024-05795-5. Commun Biol. 2024. PMID: 38278996 Free PMC article.
-
Unveiling the Immunomodulatory and regenerative potential of iPSC-derived mesenchymal stromal cells and their extracellular vesicles.Sci Rep. 2024 Oct 15;14(1):24098. doi: 10.1038/s41598-024-75956-3. Sci Rep. 2024. PMID: 39407038 Free PMC article.
-
Nrf2 activation: a key mechanism in stem cell exosomes-mediated therapies.Cell Mol Biol Lett. 2024 Mar 2;29(1):30. doi: 10.1186/s11658-024-00551-3. Cell Mol Biol Lett. 2024. PMID: 38431569 Free PMC article. Review.
-
Secretome of Mesenchymal Stromal Cells as a Possible Innovative Therapeutic Tool in Facial Nerve Injury Treatment.Biomed Res Int. 2023 Jan 14;2023:8427200. doi: 10.1155/2023/8427200. eCollection 2023. Biomed Res Int. 2023. PMID: 36691473 Free PMC article. Review.
References
-
- Katsuda T, Kosaka N, Takeshita F. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013 May;13(10-11):1637–53. - PubMed
-
- Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. - PubMed
-
- Feng Y, Yang SH, Xiao BJ. et al. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2010 Jan;46(1):32–40. - PubMed
-
- Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–9. - PubMed
-
- Chen J, Crawford R, Chen C. et al. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev. 2013 Dec;19(6):516–28. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

